Abstract
Background
Repetitive Transcranial Magnetic Stimulation (rTMS) is a novel, non-invasive method of stimulating selected regions of the brain that has both research applications and potential clinical utility, particularly for depression. In order to conduct high-quality clinical studies of rTMS, it is necessary to have a convincing placebo (or sham) treatment. Prefrontal rTMS causes cutaneous discomfort and muscle twitching; therefore, an optimal control condition, i.e., sham condition, would mimic the cutaneous sensation and muscular discomfort of rTMS without stimulating the brain. Ideally, the quality and intensity of the sham condition would feel identical to the quality and intensity of the rTMS condition except that the sham would have no effect on cortical activity. We designed and built a focal electrical stimulation system as a sham rTMS condition. While this electrical sham system is superior to methods employed in previous studies, little is known about how the new electrical sham system compares to active rTMS in terms of the level of discomfort and type of sensation it produces.
Methods
We hypothesized that the electrical sham system may not mirror the experimental condition sufficiently. We studied this hypothesis under single-blind conditions in 15 healthy adults by administering either the real or sham rTMS at high and low intensities while subjects, who were unaware of condition, rated subjective qualities of the stimulation (such as tingling, pinching, and piercing) and the painfulness of the stimuli.
Results
At low intensity stimulation, the two techniques (active and sham) differ with respect to the subjective quality of the sensation. The differences between real and sham rTMS were less dramatic at higher intensities. The best sham condition that most closely mimics real prefrontal rTMS requires individual titration of the intensity of electrical stimulation across a broad range. Performing this titration without unblinding patients is likely possible, but technically challenging. We propose a new approach to do this. It is possible to create a truly indistinguishable sham condition, but more work is needed
Introduction
Daily repeated left prefrontal repetitive Transcranial Magnetic Stimulation (rTMS) over several weeks has been found to significantly reduce depression symptoms 1–4. rTMS uses a powerful magnetic pulse that is generated by passing electricity through a metal coil placed against the head. This focused magnetic pulse passes through the skull and causes neuronal depolarization.5 In the studies using TMS as a potential antidepressant, the coil is typically targeted over the left-prefrontal cortex, which has been found to have abnormalities in functional neuroimaging studies of depression 6–8.
An important issue for developing rTMS as a treatment has been the development of different sham techniques. A commonly used sham design has been to tilt the coil either 45° or 90°. However, tilting the coil 45° was proven to stimulate the cortex, likely contributing to the variability between studies 9 and even though tilting the coil 90° does not elicit motor-evoked potentials (MEPs), this sham may feel less intense 10. An additional sham system involves using specially created rTMS sham coils, which are true rTMS coils which have an aluminum plate inserted between the coil and the scalp surface. This effectively blocks most of the magnetic field. These sham systems visually resemble the actual coil, and produce a sound during an rTMS pulse, and some percussive stimulation of the scalp. However, the active and sham rTMS coils sound different, likely produce different scalp sensations, and the sham coil does not produce superficial muscle twitching in the same manner as the active coil. Thus, although these sham coils are an improvement, they are useful only for single-blind studies as the rTMS operator quickly becomes unblinded for each patient. An ideal sham TMS system would resemble real rTMS both visually and acoustically and create a comparable cutaneous sensation without producing cortical stimulation. Mimicking the cutaneous sensation experienced during rTMS has been a challenging aspect of developing an optimal sham condition. The cutaneous sensation is caused when rTMS stimulates scalp muscles producing a twitch in the scalp or upper face that can be uncomfortable for some, painful for others.
Investigators hope that the focal electrical stimulation will create a similar sensation to rTMS without causing brain activation. This current is delivered by an electrical stimulator, which produces an electrical pulse coincident with the sham magnetic pulse. As the study launched, it was unclear what amount of electrical stimulation delivered to those receiving sham would best mimic the active rTMS treatment. Additionally, there was no way to devise a method of titrating the electrical stimulation independent of rTMS in a way that would not jeopardize the blind. After experimenting in several investigators, a 5mA, unidirectional current, medial anode placement was used for the sham design. These sham conditions were expected to safely mimic active rTMS at 120% of the individual’s motor threshold (MT).
We tested whether these sham conditions actually mimic active rTMS in terms of the perceived sensation and pain. The sham system also delivers a timed white noise that dampens out the rTMS-induced noise for both the subject and the rTMS operator. Thus the sound produced by the different coils (active and sham) is indistinguishable when wearing these earplugs. We anticipated that active and sham TMS would produce different sensations, but were unsure how we could better match the rTMS with the sham condition. We tested this using rTMS with standard intensities used in most clinical trials and different sham conditions including high/low intensities and altering anode/cathode medial placement and uni/bi-directional current.
Methods
15 healthy adults (9 men) ages 21–48 were stimulated with both real rTMS and the electrical sham control condition. Each subject was screened for a personal or family history of epilepsy 11 and signed written informed consent approved by the MUSC IRB. We used a Neuronetics system and Mecta Spectrum 5000Q (sham). To deliver the sham stimulation, one researcher triggered the electrical stimulation with a Neotonus sham coil attached, while the other researcher adjusted the appropriate settings with the RCS MECTA program. This modified ECT device is a constant current device and uses internal tests to assure that only a minimal intensity (20mA) of stimulation will be delivered to the subject, to prevent risk of seizures in the participants. The Spectrum 5000Q ECT machine delivered the electrical stimulation to the electrodes, while the Neotonus sham coil mimicked the noise and placement of the real rTMS coil and triggered the timing of the Spectrum machine. Both the real rTMS coil and the sham ran for 4 seconds at 10 Hz. Each subject received a total of 8 real TMSstimulations and 16 sham stimulations.
The subjects reclined in a supine position in the Neuronetics chair (fig 1). The forehead was swabbed with alcohol, and Pre Tac conductive skin preparation gel was placed in the general areas of electrode placement (medial and lateral). The medial electrode was centered at the nose and placed about 1cm above the eyebrows, and then the lateral electrode was placed about 1 cm to the left of the medial electrode and 1 cm above the eyebrow. The cords were placed posterior to avoid tugging and to keep the subject blinded about the anode/cathode placement. Before beginning electrical stimulation, the system was tested for static impedance with a target of less than 1000 ohms.
Figure 1.
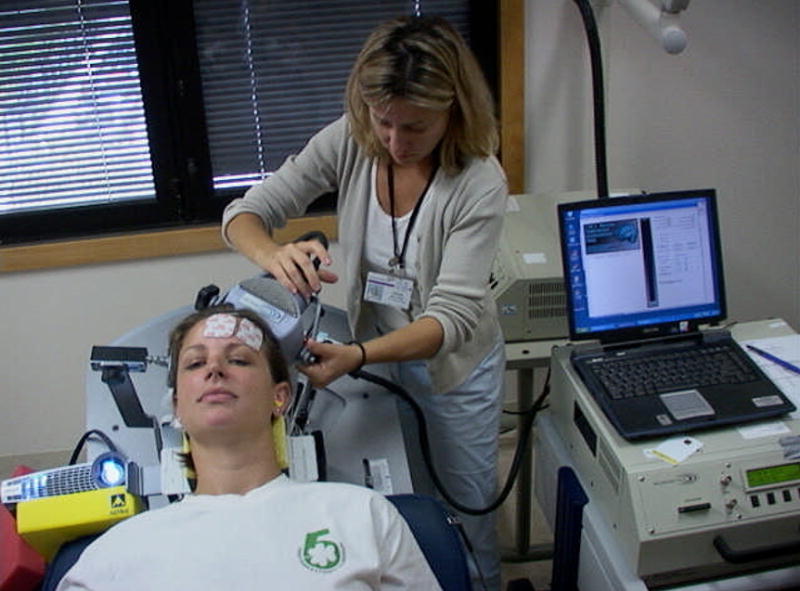
Experimental Set-up. Electrodes are placed on forehead for sham stimulation, and the TMS coil is positioned over the left-prefrontal cortex.
For electrical stimulation, we used one dose at 5mA for all subjects and then we also delivered an intensity that was about 70% (7±1/10) of the subject’s maximum pain tolerance, which we refer to in this study as ‘high intensity’ sham. This high intensity sham was found for each individual through an ascending staircase method where we started the machine at 5 mA and asked the subject to rate the pain on a scale of 0 to 10, with 0 being “no pain at all” and 10 being “the worst pain imaginable.” If the subject rated the delivered intensity below 7, the intensity was increased. If the subject rated the delivered intensity above 7, the intensity was decreased. Given the limits of this methodology, our target pain ratings were 7 ± 1. If inconsistent or unusual ratings were given, the stimulations were repeated.
The method routinely used to identify the correct area for rTMS treatment uses the motor cortex as an anatomical marker to locate the left prefrontal cortex 12. The resting motor threshold is found by exploring for the scalp location over the motor cortex area that controls right-handed muscle movement. The location is identified using .3 Hz at low intensity TMS and is verified when the TMS pulse produces movement in the right hand. Next, to find the lowest intensity required to cause a muscle twitch or movement, a computerized algorithmic system, Adaptive P.E.S.T. 3 program was used with visible movement 13. Once the motor threshold was determined, the experiment began.
The stimulations were run in a block, randomized fashion where the subject would first either receive 8 rTMS stimulations or 16 sham stimulations. All of the conditions within each of the two blocks were randomized. For rTMS, only two intensities were delivered (110% and 120% of the individual’s motor threshold). For the sham condition, each stimulation was randomized by intensity (5mA, 70% tolerance), medial placement of electrode (anode, cathode), and type of current (unidirectional, bidirectional). The combination of the three conditions was changed every two trains, giving a total of 8 combinations of stimulation, each given twice. The entire session took approximately one hour.
For each stimulation, the subject rated the sensation using a custom-developed “Face Locator” software program. This software program allows subjects to draw on a digital image of a human face where the sensation was felt. The subjects can vary the pen width to easily draw larger or smaller areas. The program records the left, right, top, and bottom boundaries of the indicated area of sensation as well as the center of the sensation on both the x and y axes. The Face Locator Program was projected on the ceiling over the Neuronetics chair, allowing the supine positioned subject to see it clearly without having to move his or her head. The subject responded to each stimulation using a mouse with the right hand. The subject drew the position and area of the sensation, and then using a visual analog scale (VAS) the subject rated the subjective qualities of the sensation (pain, tingling, sharpness, piercing, electric, tugging, pinching, and intolerability). Finally, the subject had the option of indicating whether or not the sensation had any directional quality by moving a digital compass if the sensation moved in a direction away from its origin.
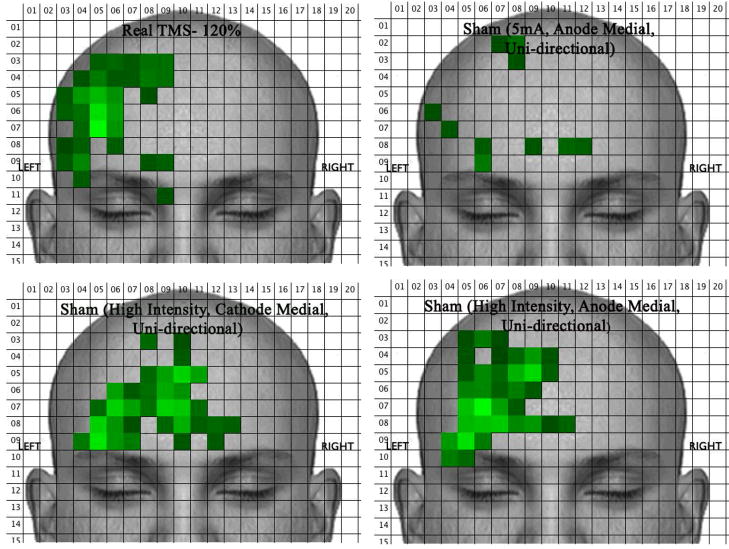
VAS data was exported into a SPSS datafile for statistical analysis. Multivariate Analysis of Variance (MANOVA) was used to evaluate the differences between real and sham TMS across all sensation ratings collected. In order to analyze data on the position and area of the sensations, a program was written to open each subject’s individual Face Locator pictures and calculate the mean number of blue (i.e., activated) pixels within each 25×25 pixel block across the entire picture. It then generates a new picture that represents average activation in 25×25 pixel blocks and represents it with different shades of green (bright green means there were many combined blue pixels within a 25×25 pixel block and dark means there were few blue pixels in a block).
Results
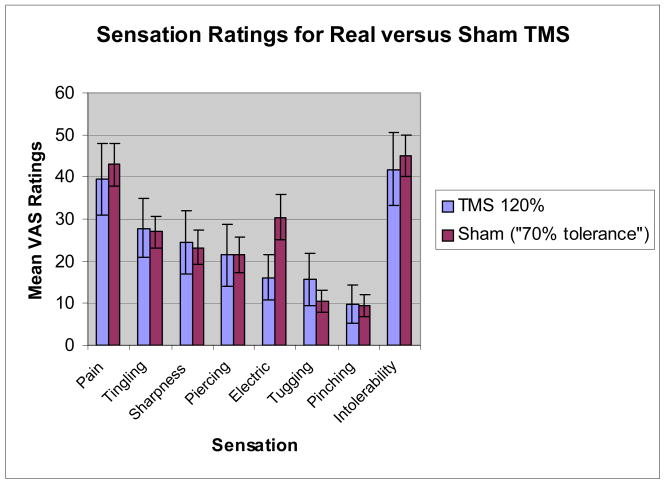
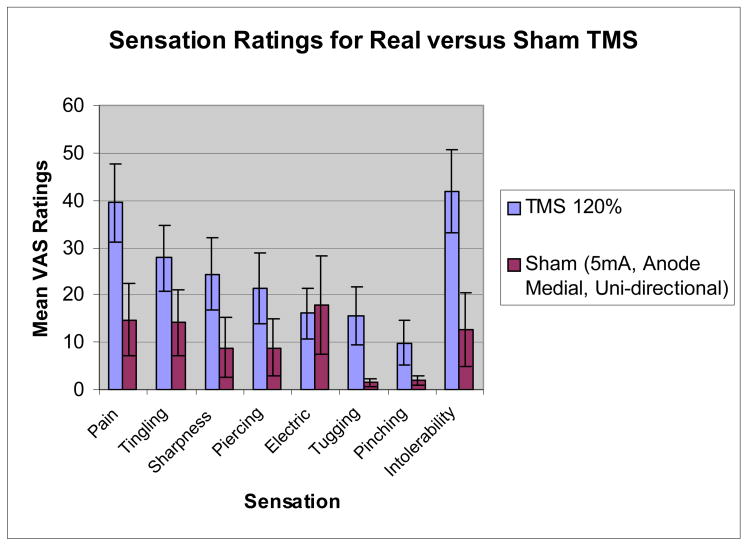
When comparing real TMS at 120% of motor threshold with sham TMS using 5mA, the anode placed medially and unidirectional current, significant differences emerged across sensation ratings (F(8,49)=8.84, p<.0001). Real TMS was rated as significantly more painful (p<.0001), tingling (p=.006), sharp (p=.002), piercing (p=.017), tugging (p=.001), pinching (p=.029), and intolerable (p<.0001) than sham TMS. Real TMS was not rated differently from sham with respect to “electrical” sensations (p=.291). When comparing real TMS at 120% with sham TMS matched to 70% of tolerable, there was no significant difference between conditions across the rated sensations (F(8,45)=1.68, p=.13).
Sham stimulation at about “70% individual pain tolerance” (high intensity sham) was significantly more painful and intolerable than at 5mA (low intensity). The location and intensity of the real rTMS (120%) is for almost all subjects, substantially more painful and larger, than the 5mA condition. Active rTMS was significantly more painful than sham at 5mA; however, these differences were less dramatic at high intensity sham. By individually titrating electrical stimulation to high intensity sham, we achieve virtually identical sensations between active rTMS and sham (Figure 3), and using unidirectional current with either cathode or anode medial placement, we achieve virtually identical sensation locations (Figure 4).
Figure 3.
Average (and 95%CI) ratings given during TMS at 120% MT compared to ratings given during sham stimulation with an intensity of about 70% individual
Figure 4.
Intensity of stimulation had the largest effect on VAS ratings and stimulation location. Changing medial polarity and current type failed to create statistically significant differences in ratings; however, results suggest that medial cathode placement and unidirectional current are slightly more painful and intolerable than medial anode placement and bi-directional current.
There were substantial individual differences in pain tolerance for the high-intensity sham stimulation. The range of sham stimulation that resulted in subject painfulness ratings of 7 out 10 was 5mA to 16mA with a mean of 10.79mA. There may be a relationship between painfulness ratings and gender/age (younger women requiring less intensity stimulation); however, with such a small sample size it is hard to draw conclusions.
Discussion
This study suggests that the sham condition with 5mA, anode medial, and unidirectional current does not produce an identical scalp sensation as real rTMS, although it is rather close and better than that used in prior trials. This sham condition, for most subjects, is both less painful and the main sensation is not felt in one concentrated area compared to active rTMS. In this study, subjects rated pain and intolerability significantly greater with rTMS than in the sham condition. This difference decreased when the sham intensity was increased to approximately 70% of the subjects’ individual pain tolerance. However, with the small sample size (15 subjects), the “70% pain tolerance” cannot be concluded as the best sham match for high intensity rTMS. Also, it is difficult to determine an individual’s “70% pain tolerance” for the sham condition without affecting the blind of a clinical rTMS study.
The key question remains: What modifications can be made to the sham to make it mimic real rTMS more closely? An ideal TMS sham needs to be as similar as possible to rTMS in order to adequately test that the cutaneous sensations of rTMS are not contributing to the ultimate outcomes of clinical, blinded studies. The preliminary findings of this study suggest that the intensity of the sham currently being used needs to be increased. However, due to great inter-individual variance in pain threshold and tolerance, either the range for sham intensity should be increased or a personalized matching system should be designed to reduce the difference between rTMS and control conditions.
To create a personalized matching system, the patient would receive alternating stimulations of rTMS and electrical stimulation at varying intensities. For each stimulation, the patient would record his pain rating on a VAS. Once the rTMS stimulation reaches the intensity of rTMS used in the trials, the P.E.S.T. program would anchor this specific pain rating. The electrical stimulations would continue until a good match is made with this anchored rTMS rating. To help keep the study double-blinded, real rTMS would continue at varying levels of intensity mixed in with electrical stimulation even after the anchored rTMS rating was found. While this would most likely bring the two sensations closer together for the individual, it might unblind the patient because they are feeling both sensations in one sitting. An alternative that would not necessarily create an ideal sham system for every individual (since the intensity would not be high enough), would be to simply increase the range of sham intensity delivered; however, personalizing the sham intensity would make for a tighter match to rTMS.
To date, no studies have been conducted to control for the pain and cutaneous sensation created by rTMS. A valid sham that is able to more closely mimic this painful sensation is needed to further evaluate the efficacy of rTMS as a possible treatment for depression and other neuropsychiatric illnesses.
Figure 2.
Graph of the average (and 95% CI) VAS ratings for various sensational qualities. Ratings given during TMS at 120% MT compared to ratings given during sham stimulation at 5mA with medial placement of anode and a unidirectional current.
Acknowledgments
The Brain Stimulation Laboratory is supported in part by the Stanley Foundation, the National Alliance for Research on Schizophrenia and Depression (NARSAD), NINDS grant RO1-AG40956, NIMH grant RO1 MH069887, 1K08MH070915-01A1 (Nahas), Borckardt,. The authors would like to thank Minnie Dobbins for administrative help.
References
- 1.Martin JLR, Barbanoj MJ, Schlaepfer TE, Clos S, Perez V, Kulisevsky J, Gironell A. The Cochrane Library. Oxford: Update Software; 2002. Transcranial magnetic stimulation for treating depression (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtzheimer PE, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacology Bulletin. 2001;35:149–169. [PubMed] [Google Scholar]
- 3.Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. Journal of Psychiatric Practice. 2002;8:270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 4.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Eisenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multi-site Randomized Controlled Trial. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2007.01.018. In Press. [DOI] [PubMed] [Google Scholar]
- 5.George MS, Belmaker RH. TMS in Clinical Psychiatry. Washington, DC: American Psychiatric Press; 2006. [Google Scholar]
- 6.Kozel FA, George MS. Neuroimaging and Depression with Inadequate Treatment Response. Primary Psychiatry. 2005;12:30–35. [Google Scholar]
- 7.Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, Herscovitch P, Post RM. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–52. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 8.George MS, Nahas Z, Li X, Kozel FA, Anderson B, Yamanaka K, Chae JH, Foust MJ. Novel treatments of mood disorders based on brain circuitry (ECT, MST, TMS, VNS, DBS) Semin Clin Neuropsychiatry. 2002;7:293–304. doi: 10.1053/scnp.2002.35229. [DOI] [PubMed] [Google Scholar]
- 9.Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: Are some “sham” forms active? Biological Psychiatry. 2000;47:325–331. doi: 10.1016/s0006-3223(99)00285-1. [DOI] [PubMed] [Google Scholar]
- 10.Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: Intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biological Psychiatry. 2001;49:460–463. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- 11.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop in the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 12.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Hallett M, Post RM. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–6. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 13.Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, Stroud Z, Nahas Z, George MS. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J Ect. 2004;20:160–5. doi: 10.1097/00124509-200409000-00007. [DOI] [PubMed] [Google Scholar]