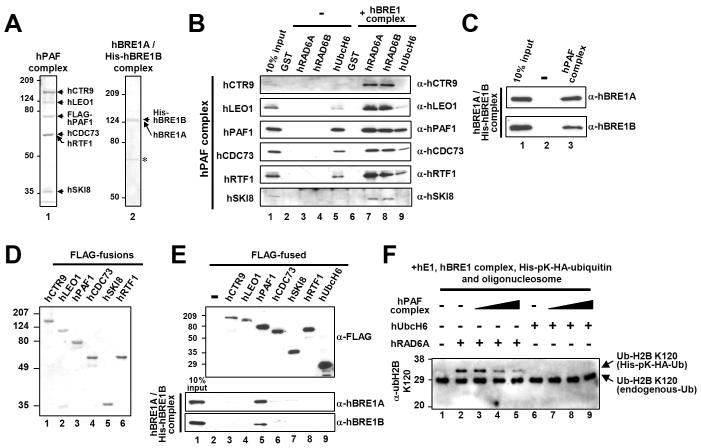
Figure 5. The hPAF Complex Directly Interacts with the hBRE1 Complex.
(A and D) Analyses of purified proteins by Coomassie blue staining. Reconstituted hPAF (isolated via FLAG-hPAF1) and hBRE1 (isolated via His-hBRE1B) complexes (A) and individual FLAG-tagged subunits of the hPAF complex (D) were expressed from baculovirus vectors. Asterisk, non-specific bands. (B, C and E) Binding of the purified hPAF complex or isolated subunits to H2B ubiquitylation factors. The purified hPAF complex was tested for binding to GST-fused E2 proteins in the presence and in the absence of the hBRE1 complex (B). The purified hBRE1 complex was tested for binding to the M2 agarose-immobilized hPAF complex (C) or to individual hPAF complex subunits (E). Bound proteins were scored by immunoblot with indicated antibodies. (F) Effect of the hPAF complex on H2B ubiquitylation. The chromatin ubiquitylation assay was performed as in Figure 4. Reactions contained 150 ng (lanes 3 and 7), 300 ng (lanes 4 and 8) or 600 ng (lanes 5 and 9) hPAF complex and hRAD6A or hUbcH6 additions as indicated.