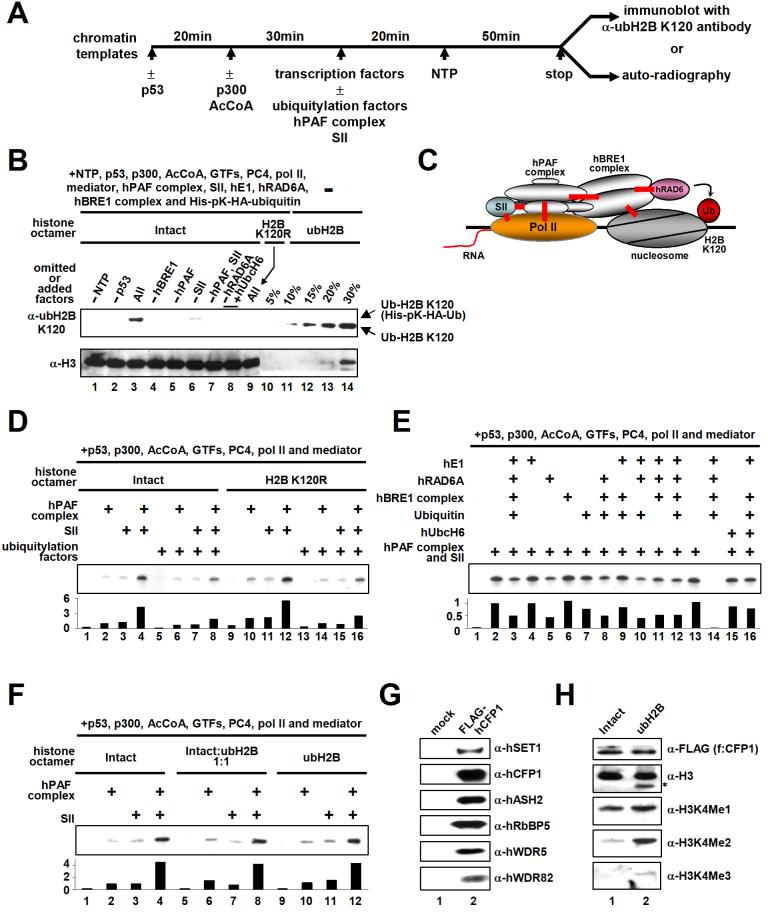
Figure 6. Transcription-dependent H2B Ubiquitylation.
(A) Schematic representation of the standard in vitro transcription assay. Transcription factors included TFIIA, TFIIB, TFIIE, TFIID, TFIIF, TFIIH, PC4, Mediator and Pol II. Ubiquitylation factors included E1, E2 (hRAD6A or hUbcH6), the hBRE1 complex and ubiquitin. Chromatin-based assays also contained the components (ACF1, ISWI, NAP1 and TOPO1) employed for chromatin assembly. Reactions contained 10 ng p53, 20 ng p300, 240 ng hPAF complex, 10 ng SII, 25 ng E1, 25 ng E2, 75 ng hBRE1 complex, and 350 ng ubiquitin where indicated. (B) Transcription-coupled H2B ubiquitylation assays with deletions and additions as indicated (lanes 1-9). In each case, nine standard transcription reactions were combined, concentrated and subjected to immunoblots with indicated antibodies. Note that all four unmodified nucleoside triphosphates were used in this assay. Histone octamers containing fully ubiquitylated H2B (indicated as % of histone octamers present in the transcription assay) were loaded in lanes 10-14. (C) Schematic model of transcription coupled-H2B ubiquitylation. Verified direct interactions are depicted by red lines. (D, E and F) Effects of H2B ubiquitylation factors (D and E) or fully ubiquitylated (semi-synthetic) H2B (F) on chromatin transcription. Relative transcription levels were quantitated by phosphoimager and normalized to that of SII alone (D and F) or to that of the hPAF complex and SII (E). (G and H) H2B ubiquitylation directly stimulates hSET1 complex-mediated H3K4 di- and tri-methylation. (G) Analyses of purified hSET1 complex (isolated via FLAG-hCFP1 by M2 agarose from a stable FLAG-hCFP1 cell line) by immunoblot. (H) Chromatin templates were assembled with unmodified H2B (lane 1) or ubH2B (lane 2) octamers and subjected to in vitro methylation assay with purified hSET1 complex. H3 methylation status was probed by indicated antibodies. The fast migrating band (asterisk) detected by an anti-H3 antibody in lane 2 is thought be N-terminus-deleted H3 because this band is not detected by H3K4 modification-specific antibodies.