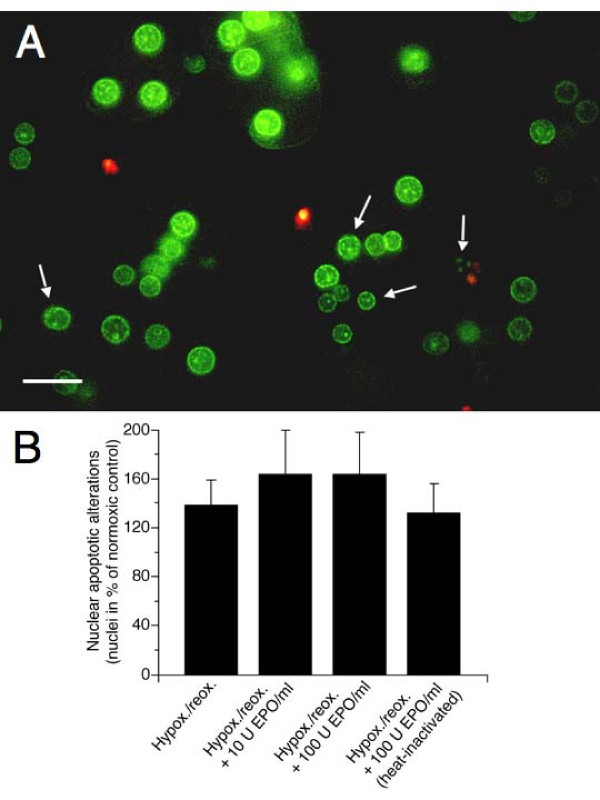
Figure 3.
Effect of rHu-EPO on the changes of nuclear morphology of cultured hepatocytes as induced by hypoxia/reoxygenation. Cultured rat hepatocytes on collagen-coated glass coverslips in 6-well cell culture plates were pre-treated or not with rHu-EPO (10 or 100 U/ml) in L-15 medium (37°C) for 20 hours. Then the cells were supplied with KH buffer with or without rHu-EPO (10 or 100 U/ml) and placed in air-tight vessels that were flushed for 10 minutes either with 74% N2/21% O2/5% CO2 or 95% N2/5% CO2. Cells were then incubated for 3 hours at 37°C. Afterwards, the buffer was removed and the cells were incubated for 24 hours in L-15 medium (37°C) again with or without rHu-EPO at 74% N2/21% O2/5% CO2. Nuclear morphology was assessed by fluorescence microscopy (λexc = 365 ± 12.5 nm, λem ≥ 515 nm; original magnification × 400) after double-staining of the cells with the membrane-permeable DNA-binding fluorochrome H33342 (1 μg/ml; green fluorescence) and the DNA-binding fluorochrome propidium iodide (5 μg/ml), that is impermeable to the intact plasma membrane but stains nuclei of necrotic and late apoptotic cells (red fluorescence). Twenty fields of vision à 15–30 hepatocytes were visually evaluated (blinded) per experiment. In (A) the effect of hypoxia/reoxygenation on the nuclear morphology is shown; bar represents 20 μm. (B) shows the effect of rHu-EPO on nuclear apoptotic alterations occurring after hypoxia/reoxygenation (hypox./reox.) in % of the normoxic controls. The microfluorographs shown are representative for three experiments with hepatocytes from different animals; bars represent means + S.D. of 3 independent experiments. Nuclear apoptotic alterations were defined as nuclear condensation, ruffling and/or fragmentation (white arrows) that had already occurred in cells that did not take up propidium iodide.