Abstract
Exposure of macrophages to transforming growth factor (TGF)-β is known to alter their functional phenotype such that antigen presentation by these cells leads to tolerance rather than an inflammatory immune response. Typically, eye-derived antigen-presenting cells (APCs) exposed to TGF-β in the local environment are known to induce a form of peripheral tolerance and protect the eye from inflammatory immune effector-mediated damage. In response to TGF-β, APCs increase their expression of tumour necrosis factor (TNF)-α and TNF receptor 2 (TNF-R2). Although TNF-α has been implicated in tolerance and the associated regulation of the inflammatory immune response, its source and the receptors involved remain unclear. In this report we determined the contribution of TNF-α and TNF-R2 expressed by TGF-β-treated APCs to their anti-inflammatory tolerogenic effect. Our results indicate that APC-derived TNF-α is essential for the ability of APCs to regulate the immune response and their IL-12 secretion. Moreover, in the absence of TNF-R2, APCs exposed to TGF-β failed to induce tolerance or regulatory cells known to participate in this tolerance. Also, blocking of TNF-R1 signalling enhanced the ability of the APCs to secrete increased TGF-β in response to TGF-β exposure. Together our results support an anti-inflammatory role of TNF-α in regulation of an immune response by TGF-β-treated APCs and suggest that TNF-R2 contributes significantly to this role.
Keywords: antigen presentation/processing, monocytes/macrophages, tolerance
Introduction
The pro-inflammatory activities of tumour necrosis factor (TNF)-α are well established. This cytokine has been implicated in various autoimmune and inflammatory diseases such as rheumatoid arthritis, Crohn's disease, multiple sclerosis and uveitis.1–4 Many treatment strategies have targeted TNF-α for interventions against inflammatory immunological processes. However, a number of reports have identified complications associated with systemic blockade of TNF-α, which include reactivation of tuberculosis, development of vasculitis and autoantibodies, and ocular inflammation.5–7 An immunosuppressive role for TNF-α has been demonstrated in autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE),8 UV-B radiation-induced suppression of contact hypersensitivity responses9 and suppression of delayed type hypersensitivity (DTH) responses to ocular antigens.10 Studies examining TNF-α-deficient animals have reported a critical role for this cytokine in regulating and limiting the extent and duration of the inflammatory response in vivo through regulation of macrophage interleukin (IL)-12 production.11 Thus TNF-α is also known for its biologically contrasting activity that negatively regulates inflammation.
The activities of TNF-α are mediated by two receptors, TNF-R1 (p55) and TNF-R2 (p75), which belong to the same family but are functionally distinct. Signalling through TNF-R1, which is considered the primary receptor, mediates the inflammatory effects of TNF-α, while TNF-R2-mediated signals contribute to effects such as thymocyte proliferation, TNF-α-mediated skin necrosis and apoptosis of activated T cells.12–15 However, the two receptors are known to trigger overlapping intracellular signalling events.16,17 Therefore, regulatory or anti-inflammatory effects of TNF-α have not yet been attributed to either one of these receptors.
Previously it has been reported that transforming growth factor (TGF)-β-exposed antigen-presenting cells (APCs) acquire the ability to induce a form of peripheral tolerance that produces a deviation in the immune response away from a T helper type 1 (Th1) response in an antigen-specific manner and results in a suppressed DTH response.18,19 This tolerance resembles that induced by eye-derived APCs that are exposed to TGF-β in the ocular environment. The tolerogenic property of TGF-β-exposed APCs was demonstrated to be dependent on TNF-α as systemic administration of neutralizing anti-TNF-α antibodies abrogated the tolerance.20 Although TGF-β-treated APCs have been shown to secrete increased levels of TNF-α, it was not clear if this APC-derived TNF-α was necessary to produce a deviation in the inflammatory immune response. Also, the mechanism by which TNF-α contributes to suppression of DTH induced by TGF-β-exposed APCs remains unclear. When we compared the transcriptional programmes of TGF-β-exposed APCs and conventional APCs we noted increased message for TNF-R2 in the former cells.21 In this study we investigated further whether APC-derived TNF-α is relevant in anti-inflammatory effects that lead to DTH suppression and if TNF-R2 plays a role in promoting such tolerogenic properties of these APCs.
Our results indicate that indeed APC-derived TNF-α is essential for the ability of APCs to produce a deviation in the immune response to achieve DTH suppression as well as to impair their secretion of IL-12, an important cytokine that supports the immune response during DTH. Similarly, TNF-R2 expressed by TGF-β-treated APCs is critical for their tolerogenic property. In the absence of this receptor, TGF-β-treated APCs fail to activate effectors with regulatory properties that are associated with their tolerance. Our observations suggest that TNF-R2 contributes significantly to the tolerance-inducing ability of TGF-β-treated APCs.
Materials and methods
Mice
TNF-R2 (p75) KO, TNF-α KO (C57BL/6 background, H-2b), C57BL/6-Tg(TcraTcrb)425Cbn/J [also known as OT-II – these are transgenic mice that express T-cell receptor (TCR) specific for chicken ovalbumin 323–339 in the context of I-Ab] and C57BL/6 (H-2b) mice, 6–8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME).
APCs
Thioglycolate-elicited peritoneal exudate cells (PECs) were used as APCs. Cells were cultured in serum-free medium: RPMI-1640 (BioWhittaker, Walkersville, MD) containing 10 mm HEPES, 0·1 mm non-essential amino acids (NEAA), 1 mm sodium pyruvate, 2mm l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin (BioWhittaker), 0·1% bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) and ITS+ culture supplement [1 μg/ml iron-free transferrin, 10 ng/ml linoleic acid, 0·3 ng/ml Na2Se and 0·2 μg/ml Fe(NO3)3] (Sigma Chemical Co). PECs were obtained from mice that had received 2 ml of a 3% thioglycolate solution (Sigma Chemical Co) intraperitoneally (i.p.) 3 days earlier.
In vitro treatment of APCs with cytokines or antibodies
APCs were cultured (1–2 × 105 cells/well) in a 96-well plate overnight with TGF-β2 (R&D Systems, Minneapolis, MN; 5 ng/ml final concentration) in serum-free culture medium and pulsed with ovalbumin (OVA) antigen (Sigma; 7 mg/ml). As previously described these APCs resemble those derived from the eye and hence the use of TGF-β2, a predominant isoform in the ocular environment.19 These APCs will be referred to as ‘TGF-β-treated APCs’ in this study. In some experiments TNF-α (PharMingen, San Diego, CA; 5 ng/ml), anti-TNF-R1 antibodies (BioLegend, San Diego, CA; 50 μg/ml) or isotype control goat immunoglobulin G (IgG; amounts matching test antibody) (R&D Systems) was added. Culture supernatants were collected at a 48-hr interval and were tested for either IL-12p70 content using an enzyme-linked immunosorbent assay (ELISA; R&D Systems) or for TGF-β levels using a bioassay. For the DTH experiments, after overnight culture, cells were cooled for 30 min at 4°, dislodged from the culture plate by vigorous pipetting, washed twice, resuspended and infused intravenously (i.v.) as indicated in each experiment.
Flow cytometry
PECs were analysed by flow cytometry to assess cell surface expression of TNF receptors or TNF-α converting enzyme (TACE). Cells were stained with phycoerythrin (PE)-labelled anti-TNF-R2, anti-TNF-R1 or anti-TACE (PharMingen). Matching isotype antibody was used as a control. For intracellular detection of tumour necrosis factor receptor-associated factor 2 (TRAF-2), cells were permeabilized (eBioscience, San Diego, CA) before staining with biotin-conjugated anti-TRAF-2 followed by PE-conjugated streptavidin. Stained cells were washed with phosphate-buffered saline (PBS) containing 0·1% BSA and analysed on a Coulter Epics XL flow cytometer with coulter system ii software (Beckman Coulter, Miami, FL).
DTH suppression assay to detect immune deviation or tolerance induction by APCs
Seven days after i.v. infusion of OVA-pulsed APCs subjected to various in vitro treatments (2-5 × 103 cells/mouse), recipients were immunized subcutaneously into the nape of the neck with OVA/complete Freund's adjuvant (CFA) (50 μg). A week later these animals received an intradermal inoculation of OVA (200 μg/20 μl) into their right ear pinna. The left ear served as an untreated control. The thickness of both ears was measured immediately before and 24 hr after the OVA injection using a micrometer (Mitutoyo 227-101; MTI Corp., Paramus, NJ). The measurements were performed in triplicate on each mouse (n = 5 per group). DTH was measured as the change in ear swelling [(24-hr measurement − 0-hr measurement in the experimental ear) − (24-hr measurement − 0-hr measurement in the untreated control ear)]. Tolerance induction or immune deviation was detected as suppression of DTH in groups infused with TGF-β-treated APCs as compared with that seen in recipients of untreated APCs. A two-tailed Student's t-test was used with significance assumed at P≤ 0·05. Results of DTH assays presented in this report are representative of two to three experiments.
In vitro co-culture of APCs and T cells
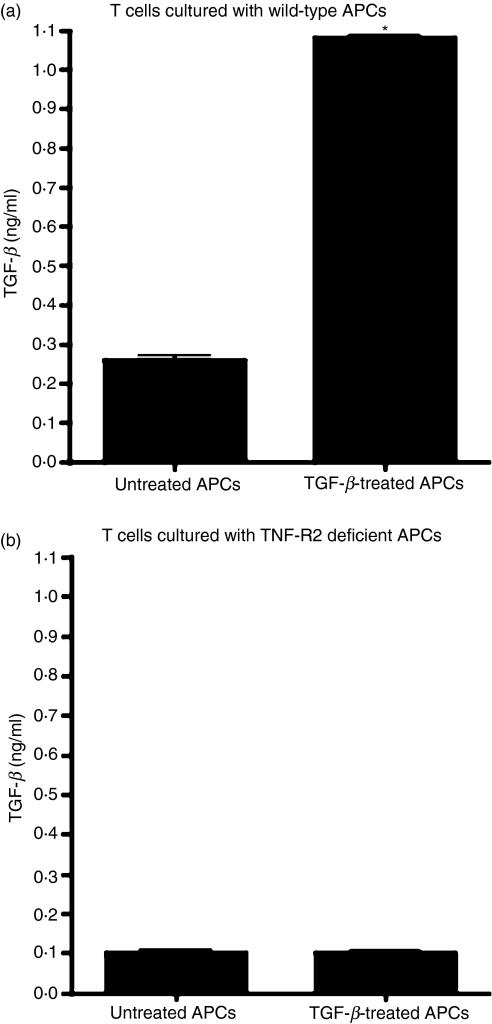
PECs (1 × 106), pretreated with or without TGF-β (5 ng/ml) in 24-well plates were cultured in 500 μl of serum-free medium with OVA (100 μg/ml). After overnight culture, the plates were washed three times with culture medium to remove TGF-β and non-adherent cells. OT-II T cells (3 × 105) were added to 24-well plates containing TGF-β-treated or untreated PECs. After 24 hr, non-adherent cells were collected, washed and cultured (1 × 106 cells/well) in 24-well plates for an additional 24-hr period and culture supernatants were collected to test for the presence of TGF-β in a bioassay.
Real-time polymerase chain reaction (PCR)
Total RNA was isolated from untreated or TGF-β-treated APCs using the RNA STAT-60 kit (Tel-Test, Inc., Friendswood, TX) according to the manufacturer's instructions. This kit utilizes a single-step method with acid guanidinium thiocyanate-phenol-chloroform extraction. cDNA was synthesized by reverse-transcribing RNA using oligodT and M-MLV RT (Promega, Madison, WI). A real-time PCR assay based on SYBR Green fluorescence was used to determine relative quantitative expression of selected genes. The sequences of the primers used for these genes were as follows: TNF-R2, forward 5′-CCC TTC AGG TTA GTG CTA AAC TC, reverse 5′-TAC AGC CCT ACC ATC CTA TAA CAG; TNF-R1, forward 5′-CCA AGT GCC ACA AAG GAA CCT ACT, reverse 5′- TGA GGT AAT TCT GGG AAG CCG TAA; TACE, forward 5′-AAG AAA GCG AGT ACA GCG TGA A, reverse 5′-CAT CAT CTC CTA TGT GGG CTA GAA; GAPDH, forward 5′-CGA GAA TGG GAA GCT TGT CA, reverse 5′-AGA CAC CAG TAG ACT CCA CGA CAT. Amplification reactions were set up using SurePRIME&GO™ Mastermix (QBiogene/MP Biomedicals, Irvine, CA). Briefly, each reaction contained 1× of mastermix, 100 μm of each dNTP, 10 mm Tris–HCl (pH 8·3), 50 mm KCl, 2·5 mm MgCl2, 0·02 U/μl SurePRIME™ DNA polymerase, 200 nm forward and 200 nm reverse primers, 1 : 20 000 SYBR green I dye (Molecular Probes Inc./Invitrogen Corp., Carlsbad, CA) and a 1 : 50 dilution of cDNA. The reactions were performed in a MicroAmp 96-well plate sealed with an optical adhesive cover (Applied Biosystems, Foster City, CA) and amplified for 40 cycles in ABI Prism® 7900HT (AME Bioscience Ltd, Toroed, Norway) with the standard PCR parameters (thermal profile: 50° for 2 min- one cycle, 95° for 15 min- one cycle, 52–55° for 1 min- 40 cycles, and 72° for 30 seconds- one cycle). Dissociation curves were analysed to ensure product specificity and amplicon identification based on Tm(melting temperature of a PCR product) values. The data generated from reactions were analysed by plotting the ΔRn fluorescence signal versus the cycle number. An arbitrary threshold was set at the midpoint of the log ΔRn (difference in the fluorescence intensity in the test reaction and baseline) versus cycle number plot. The threshold cycle (Ct) values calculated from this plot were used to determine relative quantification of gene expression by applying the comparative Ct method (ΔΔCt). ΔCt was calculated by subtracting Ct(reference gene) from Ct(target gene). The reference gene was GAPDH. The comparative expression level was then calculated by converting ΔΔCt from a logarithmic to a linear value. Fold change = 2−ΔΔCt.
Bioassay to detect TGF-β
Biologically active TGF-β was measured using Mv1Lu cells (ATCC, Rockville, MD) cultured in Eagle's minimum essential medium (EMEM) (BioWhittaker) containing 2 mm l-glutamine, 10 mm HEPES, 0·1 mm non-essential amino acids, 1 mm sodium pyruvate, 100 U/ml penicillin, 100 mg/ml streptomycin and 0·5% fetal calf serum (FCS). To measure total TGF-β (active + latent) supernatants were acid-treated (1 n HCl, 1 : 10) for 1 hr followed by neutralization with a mixture of 1 n NaOH: 1 m HEPES (1 : 1). Samples (100 μl) were added to a 96-well flat-bottom plate. Mv1Lu cells (1 × 105 cells/100 μl) were then added to each well and cultures were incubated for 72 hr at 37° in an atmosphere of 5% CO2. Cultures were pulsed with 20 μl of Cell Counting Kit-8 dye (Dojindo Molecular Technologies, Kumamoto, Japan). After 1–2 hr (at 37°, 5% CO2) cell proliferation was determined by measuring optical density in each well at 450 nm using a spectrophotometer (Bio-Tek, Winooski, VT) and half maximal inhibition was determined by polynomial regression on log-log transformation of standard curves and experimental samples.
Results
APC-derived TNF-α is essential for the tolerance induced by TGF-β-treated APCs
Intravenously infused antigen-pulsed TGF-β-treated APCs are known to induce systemic tolerance that results in deviation of an immune response away from the Th1 type.19 This tolerance is antigen specific and is attributed to regulatory T cells generated in the recipient spleen that are known to suppress Th1-mediated immune responses such as DTH or inflammatory autoimmune diseases such as uveitis. In vitro, when co-cultured with antigen-specific T cells, these APCs can also prevent interferon (IFN)-γ secretion by Th1 cells.22 Paradoxically, these APCs produce increased levels of TNF-α. Systemic administration of TNF-α neutralizing antibodies in the recipients of TGF-β-treated APCs led to a reversal of DTH suppression, indicating a critical role for TNF-α in such tolerance induction.20 However, the source of this critical TNF-α remains unclear. In our experiments we assessed if it is the APC-derived TNF-α that is essential for inducing tolerance.
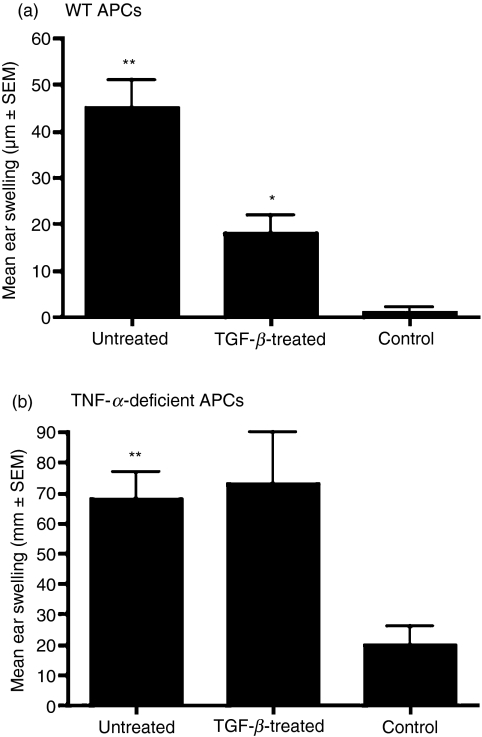
We used macrophages isolated from thioglycolate-elicited PECs as APCs. Upon overnight exposure to TGF-β, in vitro, these macrophages acquire the ability to induce antigen-specific tolerance, which suppresses a DTH response to that antigen. Experimentally this property is demonstrated when recipients infused with such APCs and subsequently immunized with antigen in complete Freund's adjuvant (CFA) fail to develop inflammation-associated local swelling at the site of antigenic challenge. Peritoneal macrophages from either wild-type or TNF-α-deficient mice were cultured with the soluble antigen OVA in the presence or absence of TGF-β. After overnight culture, these cells were harvested and infused i.v. into naïve recipients. Seven days later, these recipients were immunized with OVA in CFA. The inflammatory response in these recipients was tested a week later by injecting OVA intradermally into their ear pinnae and measuring the ear swelling response 24 hr later. A control group of animals received only OVA ear challenge. Figure 1a shows results consistent with the previously established tolerance-inducing ability of TGF-β-treated APCs. That is, while recipients of untreated wild-type APCs developed a DTH response detectable as significantly increased ear swelling compared with that detected in the control group (P < 0·05), this DTH was suppressed in the recipients of TGF-β-treated APCs (P < 0·05, compared with recipients of untreated APCs). However, as shown in Fig. 1b, TNF-α-deficient APCs treated with TGF-β failed to suppress the DTH response. These results support a critical autocrine role of TNF-α in endowing TGF-β-treated APCs with the ability to induce tolerance and suppress the inflammatory DTH response.
Figure 1.
Antigen-presenting cell (APC)-derived tumour necrosis factor (TNF)-α is essential for the induction of tolerance by transforming growth factor (TGF)-β-treated APCs. Ovalbumin-pulsed (a) wild-type (WT) or (b) TNF-α-deficient APCs cultured with or without TGF-β were injected intravenously into C57BL/6 recipients. Delayed type hypersensitivity (DTH) response was determined as described in the Materials and methods. Induction of tolerance was detected by the inhibition of DTH as determined by the decreased mean ear-swelling response in mice that received TGF-β-treated APCs compared with the recipients of untreated APCs (*P < 0·05). The ear-swelling response in recipients of untreated APCs was compared to that of the control group, which received only ear challenge (**P < 0·05).
TNF-α contributes to the inhibition of IL-12 secretion in TGF-β-treated APCs
A prototypic inflammatory cytokine such as IL-12 is known for its ability to induce pro-inflammatory Th1 responses. The ability of APCs such as macrophages and dendritic cells to secrete IL-12 correlates with their ability to deliver an immunogenic stimulus.23 The significance of the absence of IL-12 in the induction of tolerance is well supported by reports in which blocking of IL-12 secretion has led to the induction of tolerance24–26 as well as reports documenting adjuvant effects of IL-12 that interrupt tolerance induction.27,28 Previously it was demonstrated that TGF-β-treated APCs are impaired in their ability to secrete IL-12, and this deficiency is accompanied by decreased expression of CD40, ligation of which is known to induce IL-12 secretion.29 The decreased IL-12 synthesis by TGF-β-treated APCs is consistent with their ability to induce peripheral tolerance and suppress the Th1-mediated DTH response. Some reports have shown that IL-12 production by macrophages can be suppressed by TNF-α.11,30 As TGF-β exposure of APCs was reported to increase their TNF-α synthesis, we examined the possibility that reduced IL-12 secretion in these APCs is dependent on TNF-α.
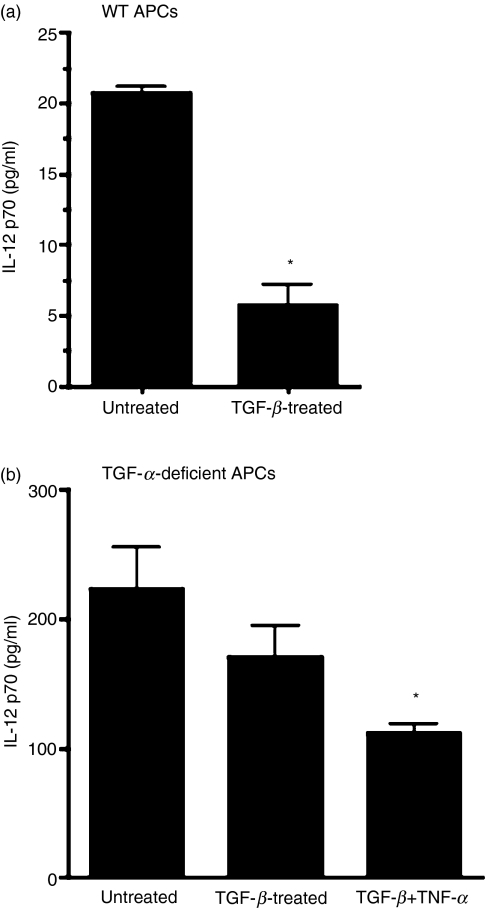
To examine this possibility, we cultured thioglycolate-elicited PECs derived from either wild-type or TNF-α-deficient mice in the presence of antigen (OVA) and with or without TGF-β. In some cultures of TNF-α-deficient APCs with TGF-β, TNF-α was provided exogenously. Culture supernatants were collected after overnight incubation of cells at 37° in 5% CO2. These supernatants were tested for their IL-12 content using ELISA. As expected, culture supernatants from wild-type APCs exposed to TGF-β contained significantly reduced levels of IL-12 (Fig. 2a). However, such a decrease was not detectable in TNF-α-deficient APCs treated with TGF-β (Fig. 2b). Exogenously added TNF-α, however, permitted significant IL-12 inhibition in TGF-β-treated TNF-α-deficient APCs. These results indicate that TNF-α secreted by TGF-β-treated APCs contributes to reduced synthesis of IL-12 and thereby supports their ability to induce peripheral tolerance and DTH suppression.
Figure 2.
Tumour necrosis factor (TNF)-α contributes to impaired interleukin (IL)-12 secretion by transforming growth factor (TGF)-β-treated antigen-presenting cells (APCs). Culture supernatants were collected from untreated APCs or those treated with TGF-β (5 ng/ml) and/or TNF-α (5 ng/ml) as described in the Materials and methods. These APCs were harvested from either (a) wild-type (WT) or (b) TNF-α-deficient mice. The amount of IL-12 in culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA) (*P < 0·05 compared with untreated control).
TGF-β-treated APCs increase expression of TNF-R2 but not TNF-R1
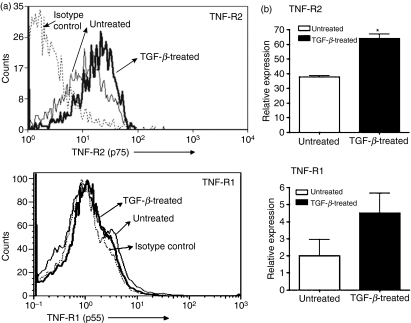
Of the two TNF receptors, most of the pro-inflammatory activities of TNF-α are associated with TNF-R1, and signalling via TNF-R2 is considered potentially immunoregulatory.8,9,31,32 Experiments conducted using mice deficient in either of the TNF receptors have highlighted their different functional roles. In microarray analysis, macrophage hybridoma cells which are functionally equivalent to primary macrophages were found to up-regulate their expression of TNF-R2 when treated with TGF-β. No significant change was detected in the expression of TNF-R1. Similar to primary macrophages these hybridoma cells acquire a tolerance-inducing property upon their exposure to TGF-β.19 This increased selective expression of TNF-R2 in tolerogenic APCs is consistent with its possible immunoregulatory role. We further confirmed our observations in the microarray analysis by real-time PCR (data not shown). To ensure that increased message for TNF-R2 in TGF-β-treated APCs is reflected in the associated protein levels in these APCs, we assessed cell surface expression of TNF-R1 and TNF-R2 on APCs by flow cytometry. Untreated and TGF-β-treated thioglycolate-elicited PECs were stained with fluorescent label-conjugated anti-TNF-R1, anti-TNF-R2 or isotype control antibody. As shown in Fig. 3a we detected increased levels of TNF-R2 but not TNF-R1 on the cell surface in TGF-β-treated APCs. We also confirmed that TGF-β-treated primary macrophages derived from these PECs increase their expression of TNF-R2 but not TNF-R1 using real-time PCR analysis (Fig. 3b). Similar to hybridoma cells, macrophages from PECs exhibit selectively increased expression of TNF-R2 in response to TGF-β treatment. Based on these results we speculate that increased expression of TNF-R2 is likely to be important in attributing a critical role to TNF-α in inducing tolerance.
Figure 3.
Tumour necrosis factor receptor 2 (TNF-R2) expression is increased in TGF-β-treated antigen-presenting cells (APCs). (a) APCs were cultured overnight in the presence of medium alone or transforming growth factor (TGF)-β2 (5 ng/ml). Harvested cells were stained with phycoerythrin (PE)-labelled anti-TNF-R2 or anti-TNF-R1 or isotype control antibodies and analysed by flow cytometry. (b) Total RNA isolated from untreated or TGF-β2 (5 ng/ml)-treated APCs was subjected to SYBR green real-time polymerase chain reaction (PCR) analysis as described in the Materials and methods using specific primers for TNF-R2, TNF-R1 and the housekeeping gene GAPDH. Message levels for TNF-R2 and TNF-R1 are presented as expression relative to GAPDH (*P < 0·05 compared with message level in untreated APCs).
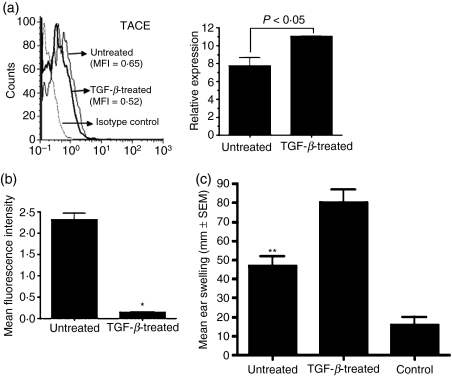
While the soluble form of TNF-α predominantly signals via TNF-R1,33 it is known that the membrane-bound form of TNF-α activates TNF-R2.34 A metalloproteinase TACE is responsible for the proteolytic cleavage of transmembrane TNF-α as well as both the receptors to release them in a soluble form.35 To determine whether increased expression of TACE in TGF-β-treated APCs might explain the lack of a pro-inflammatory effect of TNF-α by virtue of its ability to generate increased numbers of soluble TNF receptors capable of neutralizing soluble TNF-α, we compared expression of TACE in untreated and TGF-β-treated APCs using real-time PCR to detect the message and flow cytometry to detect its cell surface expression. As shown in Fig. 4a, although there was no significant change in the message for TACE, the cell surface expression of this enzyme was reduced in TGF-β-treated APCs compared with that detected on untreated APCs. These results suggest that TGF-β alters TACE expression, possibly by regulating its post-transcriptional modification, and that reduced expression of this enzyme rules out generation of increased numbers of soluble TNF receptors. It also suggests a possible increase in the expression of membrane-bound TNF-α which is known to primarily activate TNF-R2. Activation of TNF-R2 is associated with cytosolic depletion of a signalling adaptor protein, TRAF-2.36 We therefore next assessed cytosolic TRAF-2 levels in TGF-β-treated APCs to determine whether TNF-R2 on these cells was engaged. For this purpose, untreated and TGF-β-treated APCs were permeabilized and stained with fluorescent label-conjugated anti-TRAF-2 antibodies followed by flow cytometric analysis. As shown in Fig. 4b, cytosolic TRAF-2 was indeed depleted in TGF-β-treated APCs compared with the untreated APCs, suggesting TNF-R2 engagement in these cells.
Figure 4.
Tumour necrosis factor receptor 2 (TNF-R2) is essential for the ability of transforming growth factor (TGF)-β-treated antigen-presenting cells (APCs) to induce immune deviation. (a) Expression of TNF-α converting enzyme (TACE) was detected on the cell surface of untreated or TGF-β-treated APCs by flow cytometry using phycoerythrin (PE)-conjugated anti-TACE antiobody (left panel) and total RNA harvested from these cells was analysed by real-time polymerase chain reaction (PCR) to measure message levels for TACE as described in the Materials and methods. Message levels are presented as expression relative to GAPDH (right panel). (b) Intracellular levels of tumour necrosis factor receptor-associated factor 2 (TRAF-2) were determined in untreated or TGF-β-treated APCs by staining permeabilized cells with biotin-conjugated anti-TRAF-2 followed by PE-conjugated streptavidin. The mean fluorescence intensity (MFI) of staining is presented (*P < 0·05 compared with MFI in untreated APCs). (c) C57BL/6 recipients were infused intravenously with untreated or TGF-β-treated APCs derived from TNF-R2-deficient mice. Immune deviation was determined using a delayed type hypersensitivity (DTH) assay and by comparing the mean ear-swelling response in mice that received TGF-β-treated APCs with that detected in the recipients of untreated APCs (P > 0·05). The ear-swelling response in the recipients of untreated APCs was compared to that of the control group which received only ear challenge (**P < 0·05).
TNF-R2-deficient APCs treated with TGF-β fail to induce peripheral tolerance
As depicted in Fig. 1a, TGF-β-treated APCs of wild-type mice induce peripheral tolerance that suppresses Th1-mediated immune responses such as DTH. To determine the significance of TNF-R2 in this ability of APCs to induce peripheral tolerance, we used thioglycolate-elicited PEC-derived macrophages from TNF-R2-deficient mice as APCs. These cells were pulsed with OVA in the presence or absence of TGF-β and cultured overnight at 37°. Two groups of wild-type mice were infused i.v. with these OVA-pulsed APCs, while a third group that did not receive any cells served as a control. One week later, the two experimental groups (and not the control group) that received APCs were immunized subcutaneously with OVA in CFA and 7 days later all three groups were tested for their DTH response to an ear challenge with OVA at 24-hr intervals. As shown in Fig. 4c, the ear-swelling response was minimal in the control group, indicating an absence of DTH response. A significantly increased ear swelling response in recipients of untreated APCs was consistent with that seen in wild-type recipients (Fig. 1a). However, unlike the TGF-β-treated wild-type APCs, TNF-R2-deficient APCs treated with TGF-β did not suppress the DTH response. This failure to suppress DTH is indicated by the increased ear-swelling response in the recipients of untreated as well as TGF-β-treated TNF-R2-deficient APCs. In fact the ear-swelling response detected in the latter group was significantly increased compared with that in the former group. This was probably a result of the enhanced inflammatory effects of TNF-α secreted by these APCs mediated via TNF-R1 in the absence of TNF-R2. These results support a significant role played by TNF-R2 in permitting tolerogenic activity of TGF-β-treated APCs.
Failure to induce peripheral tolerance of TNF-R2-deficient APCs treated with TGF-β correlates with their activation of effector T cells with an altered phenotype
The deficiency in acquiring a tolerogenic phenotype in the absence of TNF-R2 was further confirmed by examining effector T cells activated by these APCs in an in vitro assay. Previously it was demonstrated that OVA-specific T cells activated by OVA-pulsed TGF-β-treated APCs either in vivo or in vitro exhibit regulatory properties and can prevent inflammation in an antigen-specific manner by secreting their own TGF-β.37,38 Neutralization of this TGF-β interfered with the regulatory ability of these T cells, demonstrating TGF-β-dependent regulation of inflammatory effectors. We therefore compared the TGF-β-secreting abilities of OVA-specific T cells co-cultured with untreated or TGF-β-treated APCs isolated either from wild-type or TNF-R2-deficient mice. Thioglycolate-elicited macrophages derived from PECs of wild-type or TNF-R2-deficient mice were pulsed with OVA and cultured overnight at 37° in the presence or absence of TGF-β. After overnight culture, adherent cells were rinsed with freshly prepared cultured medium without any TGF-β. These cells were then co-cultured with OVA-specific T cells derived from OT-II mice at 37° in serum-free culture medium. After 24 hr of co-culture, these T cells were harvested, washed with medium and cultured in a 96-well plate in freshly prepared serum-free medium. Culture supernatants collected from these cultures 24 hr later were analysed for the presence of TGF-β using a bioassay. As shown in Fig. 5a, T cells co-cultured with TGF-β-treated wild-type APCs produced significantly increased amounts of total TGF-β (as reported previously), indicating their regulatory phenotype. However, T cells co-cultured with TGF-β-treated TNF-R2-deficient APCs failed to produce increased levels of total TGF-β (Fig. 5b). Therefore, the phenotype of these T cells was altered and was deficient in a key feature of regulatory cells, thus reflecting their potential lack of regulatory properties. These results are consistent with the failure of these TNF-R2-deficient APCs to suppress the inflammatory DTH response after TGF-β exposure. Together these results suggest that TNF-R2 expressed by TGF-β-treated APCs plays a significant role in generating regulatory T cells that participate in suppressing the inflammatory DTH response and inducing peripheral tolerance.
Figure 5.
Expression of tumour necrosis factor receptor 2 (TNF-R2) by transforming growth factor (TGF)-β-treated antigen-presenting cells (APCs) supports a regulatory phenotype of effector T cells. Transgenic ovalbumin (OVA)-specific T cells were co-cultured with OVA-pulsed (a) wild-type (WT) or (b) TNF-R2-deficient APCs that were either untreated or TGF-β-treated (see Materials and methods). Activated T cells were washed and transferred to a fresh well and total TGF-β released in culture supernatants in 24 hr was measured using a bioassay (*P < 0·05 compared with T cells co-cultured with untreated APCs).
TNF-R2 on APCs contributes to increased TGF-β secretion of tolerogenic APCs
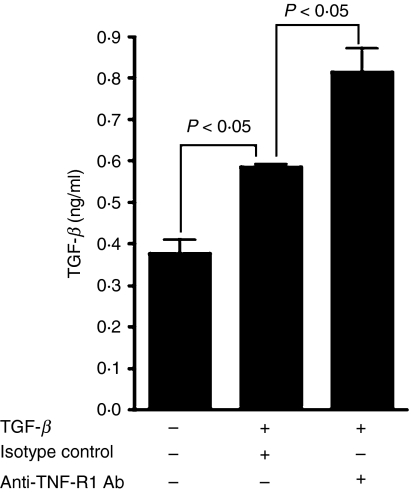
Previously it was demonstrated that APCs exposed to TGF-β produced increased levels of TGF-β message as well as protein and that their ability to induce regulatory T cells capable of suppressing the inflammatory response depended on this increased synthesis of TGF-β.22 Our results so far indicate that the effectors activated by TGF-β-treated APCs in the absence of TNF-R2 do not exhibit a typical regulatory phenotype. To determine whether TNF-R2 on APCs contributes to their ability to modulate their TGF-β secretion, we chose to address whether signals mediated by this receptor can enhance TGF-β secretion in tolerogenic APCs. We were unable to test this by directly activating TNF-R2 using agonist antibody in our experiments because such an antibody for the murine receptor was not available. We therefore attempted an indirect approach of blocking TNF-R1-mediated signals. Untreated APCs or those treated with TGF-β in the presence or absence of TNF-R1 blocking antibody were cultured overnight (antibody concentrations used were 50 times higher than the reported concentration required to attain 50% blockade of receptors) followed by thorough washes with culture medium to remove TGF-β. Adherent cells were further cultured in serum-free medium for 24 hr and culture supernatants collected from these cultures were analysed for their total TGF-β content in a bioassay. As indicated by the results in Fig. 6, the presence of isotype control antibody did not interfere with the increased levels of total TGF-β released by TGF-β-treated APCs and these levels were further enhanced in the presence of TNF-R1 blocking antibody. A similar experiment using TNF-R2 blocking antibody did not result in any change in the total TGF-β produced by TGF-β-treated APCs (data not shown). These results suggest that signals mediated through TNF-R2 are supportive of the ability of TGF-β-treated APCs to produce more TGF-β, a property important for their ability to induce regulatory cells and tolerance. Therefore an increased expression of TNF-R2 on such APCs appears to correlate with their tolerance-inducing property. Furthermore, these results indicate a possible reason behind the altered phenotype of effectors activated by TGF-β-treated TNF-R2-deficient APCs. Together these data support the possibility that signals mediated through TNF-R2 support anti-inflammatory/tolerogenic properties of TGF-β-treated APCs.
Figure 6.
Tumour necrosis factor receptor 2 (TNF-R2)-mediated signals contribute to increased transforming growth factor (TGF)-β secretion by tolerance-inducing TGF-β-treated antigen-presenting cells (APCs). Culture supernatants were collected at 24-hr intervals from APCs cultured in the absence or presence of TGF-β and anti-TNF-R1 blocking antibody or isotype control antibody as described in the Materials and methods. The total TGF-β content of these supernatants was determined using a bioassay.
Discussion
TGF-β exposure of APCs such as macrophages in the ocular environment confers upon them the ability to induce a form of peripheral tolerance that suppresses inflammatory immune responses. This is accomplished by generating regulatory T cells that are antigen specific. Tolerogenic TGF-β-exposed APCs have been shown to suppress inflammation in autoimmune diseases such as uveitis and EAE.19,39 Previously, on the basis of differential expression of genes in APCs exposed to TGF-β compared with untreated controls, we identified TNF-R2 (p75) as one of the genes up-regulated in TGF-β-treated APCs. In this study we report a significant contribution by TNF-R2 to anti-inflammatory or tolerizing properties of such APCs.
In response to TGF-β treatment APCs produce increased levels of TNF-α, and systemic neutralization of this typically pro-inflammatory cytokine led to a loss of tolerance induced by such APCs.10,20 These observations suggested an anti-inflammatory role of TNF-α that is consistent with recent reports on its regulatory potential in inflammation.8,11,30,40 During an inflammatory response, effector T cells are also known to secrete TNF-α. Therefore, loss of tolerance by systemic neutralization of TNF-α did not address the significance of TNF-α expressed specifically by TGF-β-treated APCs. Our results in this study suggest attribution of such an anti-inflammatory role to APC-derived TNF-α, as cells deficient in TNF-α failed to induce tolerance when exposed to TGF-β. It was previously demonstrated that TGF-β inhibits expression of accessory molecules, including IL-12, in APCs, thereby interrupting their ability to initiate or support a typical Th1 response.29 Our results indicate that, in the absence of TNF-α, regulation of IL-12 secretion is impaired, as TNF-α-deficient APCs express significantly higher levels of IL-12, and that their exposure to TGF-β does not inhibit this IL-12 expression unless TNF-α is provided exogenously. These results clearly indicate that APC-derived TNF-α contributes to the impaired IL-12 expression reported previously in TGF-β-treated APCs. Similar TNF-α-dependent modulation of IL-12 synthesis was reported by others and was associated with the role of TNF-α in terminating an inflammatory response against a bacterial infection.40 In our experiments, lack of tolerance induction by TNF-α-deficient TGF-β-treated APCs is consistent with their impaired ability to regulate IL-12. Our results therefore support an anti-inflammatory role of TNF-α in tolerance induced by TGF-β-treated APCs.
Although both TNF-R1 and TNF-R2 are expressed on macrophages, a selective increase in the expression of TNF-R2 in tolerogenic macrophages suggested a possible association of this receptor with the anti-inflammatory effect of TNF-α. Unlike TNF-R1, TNF-R2 is incapable of activating the mitogen-activated protein kinase (MAPK) pathway and is also known to be less efficient in activating the nuclear factor (NF)-κB pathway.41–43 Taking into account such differences, it is conceivable that a relative change in the expression of one of the two receptors on the TNF-α target cell can change the physiological effect of TNF-α. This possibility is supported by our results, as the absence of TNF-R2 signalling interfered with the tolerogenic property of APCs. In fact, our results indicate that, in the absence of TNF-R2, APC-derived TNF-α exerts a more pro-inflammatory effect, presumably by predominant activation of TNF-R1. These results are consistent with the observations reported by others that TNF-R1-mediated signalling in TNF-R2 knock-out cells induces nitric oxide production.44,45 In our experiments, APCs are treated overnight with TGF-β and during this period they acquire tolerogenic properties. Therefore, TNF-α produced by these cells may act in either an autocrine or a paracrine manner. Consistent with the reduced TACE expression on TGF-β-exposed APCs, we noted enhanced cell surface expression of TNF-α in these cells compared with untreated cells by flow cytometry (data not shown). It has been reported that the membrane-bound form of TNF-α is far superior to soluble TNF-α in activating TNF-R2.34 Therefore, it is quite possible that small amounts of membrane-bound TNF-α on TGF-β-exposed APCs predominantly activate TNF-R2 on the cells in the vicinity. It has been shown that stimulation of TNF-R2 leads to recruitment of an adaptor protein, TRAF-2, thus resulting in depletion of cytosolic TRAF-2.36 Indeed, such depletion of cytosolic TRAF-2 was detectable in TGF-β-exposed APCs, confirming ligation of TNF-R2 on these APCs. Therefore, increased TNF-R2 expression and its requirement for tolerance induction by TGF-β-treated APCs suggest that increased relative signalling via this TNF receptor contributes significantly to an anti-inflammatory role of TNF-α secreted by these APCs.
Two significant properties of TGF-β-exposed APCs are currently thought to contribute to their anti-inflammatory effect. One of these is their impaired IL-12 secretion and the other their enhanced ability to secrete TGF-β.22 Previously we have reported that impaired IL-12 secretion by APCs is not adequate for their tolerance-inducing ability.46 Type I IFN (IFN-β) secreted by TGF-β-exposed APCs, although it can impair IL-12 secretion, could not confer a tolerance-inducing property on APCs directly exposed to IFN-β. We did not detect any significant alteration in IL-12 impairment of TGF-β-treated APCs in the absence of TNF-R2-mediated signals (data not shown). Therefore, although TNF-α regulates IL-12 secretion by APCs, this effect appears to be independent of their TNF-R2 expression. However, we noted a significant enhancement in TGF-β secretion in the presence of TNF-R1 blocking antibody. Also, in the absence of TNF-R2, TGF-β-treated APCs failed to activate the regulatory phenotype of effector T cells. This phenotype is known be dependent on TGF-β secreted by APCs. Thus TNF-R2-mediated signals appear to regulate TGF-β secretion by APCs, which supports the development of regulatory T cells and associated immunological tolerance. Therefore, increased expression of TNF-R2 in TGF-β-exposed APCs correlates with their tolerogenic property. These results suggest that an altered balance in relative signalling mediated by the two TNF receptors in TGF-β-treated APCs contributes to their ability to induce tolerance. Although signalling events associated with TNF-R2 are not yet directly linked to the expression of TGF-β, based on our results such a possibility cannot be ruled out.
Taken together, our results demonstrate that both TNF-α and TNF-R2 expressed by TGF-β-treated APCs are critical to their ability to induce tolerance and suppress the inflammatory response. While TNF-α exerts its anti-inflammatory effect by contributing to impaired IL-12 synthesis in TGF-β-treated APCs, TNF-R2 contributes partly by enhancing the TGF-β secretion of APCs. Furthermore, these results suggest that the distribution of TNF receptors on target cells may dictate the physiological effect of TNF-α. Our observations highlight a beneficial effect of TNF-α in tolerance induction and suggest that interruption of this mechanism during systemic TNF-α blockade may be the basis of some complications reported in anti-TNF therapies. These results therefore indicate the need to modify attempts to disable TNF-α function in therapeutic approaches in such a way as to spare the tolerogenic properties of APCs.
Acknowledgments
This research was supported by National Institute of Health grant EY015472. The authors are grateful for the suggestions and guidance provided by the late Dr J. Wayne Streilein, which contributed to the initiation of these experiments.
Glossary
Abbreviations:
- DTH
delayed type hypersensitivity
- EAE
experimental autoimmune encephalomyelitis
- PEC
peritoneal exudate cells
- TRAF2
tumour necrosis factor receptor-associated factor 2
References
- 1.Brennan FM, Maini RN, Feldmann M. TNF alpha–a pivotal role in rheumatoid arthritis? Br J Rheumatol. 1992;31:293. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- 2.Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, Targan SR. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997;159:6276. [PubMed] [Google Scholar]
- 3.Navikas V, Link H. Review: cytokines and the pathogenesis of multiple sclerosis. J Neurosci Res. 1996;45:322. doi: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Hale S, Lightman S. Anti-TNF therapies in the management of acute and chronic uveitis. Cytokine. 2006;33:231. doi: 10.1016/j.cyto.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis. 2005;41(Suppl 3):S199. doi: 10.1086/429998. [DOI] [PubMed] [Google Scholar]
- 6.Jarrett SJ, Cunnane G, Conaghan PG, Bingham SJ, Buch MH, Quinn MA, Emery P. Anti-tumor necrosis factor-alpha therapy-induced vasculitis: case series. J Rheumatol. 2003;30:2287. [PubMed] [Google Scholar]
- 7.Kassiotis G, Kollias G. TNF and receptors in organ-specific autoimmune disease: multi-layered functioning mirrored in animal models. J Clin Invest. 2001;107:1507. doi: 10.1172/JCI13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001;193:427. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurimoto I, Streilein JW. Tumor necrosis factor-alpha impairs contact hypersensitivity induction after ultraviolet B radiation via TNF-receptor 2 (p75) Exp Dermatol. 1999;8:495. doi: 10.1111/j.1600-0625.1999.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson TA, Herndon JM, Dube P. The immune response and the eye: a role for TNF alpha in anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 1994;35:2643. [PubMed] [Google Scholar]
- 11.Hodge-Dufour J, Marino MW, Horton MR, et al. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:13806. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehan KC, Pinckard JK, Arthur CD, Dehner LP, Goeddel DV, Schreiber RD. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J Exp Med. 1995;181:607. doi: 10.1084/jem.181.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, Palladino MA., Jr Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol. 1993;151:4637. [PubMed] [Google Scholar]
- 14.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 15.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943. [PubMed] [Google Scholar]
- 16.Vandenabeele Peter DW. Beyaert Rudi, Fiers Walter Two tumor necrosis factor receptors:structure and function. Trends Cell Biol. 1995;5:392. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 17.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular transforming growth factor-beta. Eur J Immunol. 1992;22:165. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- 19.Hara Y, Caspi RR, Wiggert B, Dorf M, Streilein JW. Analysis of an in vitro-generated signal that induces systemic immune deviation similar to that elicited by antigen injected into the anterior chamber of the eye. J Immunol. 1992;149:1531. [PubMed] [Google Scholar]
- 20.Hecker KH, Niizeki H, Streilein JW. Distinct roles for transforming growth factor-beta2 and tumour necrosis factor-alpha in immune deviation elicited by hapten-derivatized antigen-presenting cells. Immunology. 1999;96:372. doi: 10.1046/j.1365-2567.1999.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masli S, Turpie B, Hecker KH, Streilein JW. Expression of thrombospondin in TGFbeta-treated APCs and its relevance to their immune deviation-promoting properties. J Immunol. 2002;168:2264. doi: 10.4049/jimmunol.168.5.2264. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi M, Kosiewicz MM, Alard P, Streilein JW. On the mechanisms by which transforming growth factor-beta 2 alters antigen-presenting abilities of macrophages on T cell activation. Eur J Immunol. 1997;27:1648. doi: 10.1002/eji.1830270709. [DOI] [PubMed] [Google Scholar]
- 23.Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol. 2006;177:1618. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- 24.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J Immunol. 1996;157:2348. [PubMed] [Google Scholar]
- 25.Marth T, Zeitz Z, Ludviksson B, Strober W, Kelsall B. Murine model of oral tolerance. Induction of Fas-mediated apoptosis by blockade of interleukin-12. Ann N Y Acad Sci. 1998;859:290. doi: 10.1111/j.1749-6632.1998.tb11148.x. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Lu L, Wang Z, Wang L, Fung JJ, Thomson AW, Qian S. Il-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J Immunol. 2001;166:5619. doi: 10.4049/jimmunol.166.9.5619. [DOI] [PubMed] [Google Scholar]
- 27.Van Parijs L, Perez VL, Biuckians A, Maki RG, London CA, Abbas AK. Role of interleukin 12 and costimulators in T cell anergy in vivo. J Exp Med. 1997;186:1119. doi: 10.1084/jem.186.7.1119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Muller G, Saloga J, Germann T, Schuler G, Knop J, Enk AH. IL-12 as mediator and adjuvant for the induction of contact sensitivity in vivo. J Immunol. 1995;155:4661. [PubMed] [Google Scholar]
- 29.Takeuchi M, Alard P, Streilein JW. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J Immunol. 1998;160:1589. [PubMed] [Google Scholar]
- 30.Ma X, Sun J, Papasavvas E, et al. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol. 2000;164:1722. doi: 10.4049/jimmunol.164.4.1722. [DOI] [PubMed] [Google Scholar]
- 31.Eugster HP, Frei K, Bachmann R, Bluethmann H, Lassmann H, Fontana A. Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur J Immunol. 1999;29:626. doi: 10.1002/(SICI)1521-4141(199902)29:02<626::AID-IMMU626>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann R, Eugster HP, Frei K, Fontana A, Lassmann H. Impairment of TNF-receptor-1 signaling but not fas signaling diminishes T-cell apoptosis in myelin oligodendrocyte glycoprotein peptide-induced chronic demyelinating autoimmune encephalomyelitis in mice. Am J Pathol. 1999;154:1417. doi: 10.1016/S0002-9440(10)65395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:570. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grell M, Douni E, Wajant H, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 35.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 36.Fotin-Mleczek M, Henkler F, Samel D, et al. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- 37.Kezuka T, Streilein JW. Analysis of in vivo regulatory properties of T cells activated in vitro by TGFbeta2-treated antigen presenting cells. Invest Ophthalmol Vis Sci. 2000;41:1410. [PubMed] [Google Scholar]
- 38.Kezuka T, Streilein JW. In vitro generation of regulatory CD8 + T cells similar to those found in mice with anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 2000;41:1803. [PubMed] [Google Scholar]
- 39.Faunce DE, Terajewicz A, Stein-Streilein J. Cutting edge: in vitro-generated tolerogenic APC induce CD8+ T regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J Immunol. 2004;172:1991. doi: 10.4049/jimmunol.172.4.1991. [DOI] [PubMed] [Google Scholar]
- 40.Marino MW, Dunn A, Grail D, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jupp OJ, McFarlane SM, Anderson HM, Littlejohn AF, Mohamed AA, MacKay RH, Vandenabeele P, MacEwan DJ. Type II tumour necrosis factor-alpha receptor (TNFR2) activates c-Jun N-terminal kinase (JNK) but not mitogen-activated protein kinase (MAPK) or p38 MAPK pathways. Biochem J. 2001;359:525. doi: 10.1042/0264-6021:3590525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McFarlane SM, Jupp OJ, Cobban HJ, Hunter I, Anderson HM, Vandenabeele P, Nixon GF, MacEwan DJ. Stimulation of stress-activated but not mitogen-activated protein kinases by tumour necrosis factor receptor subtypes in airway smooth muscle. Biochem Pharmacol. 2001;61:749. doi: 10.1016/s0006-2952(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 43.McFarlane SM, Pashmi G, Connell MC, Littlejohn AF, Tucker SJ, Vandenabeele P, MacEwan DJ. Differential activation of nuclear factor-kappaB by tumour necrosis factor receptor subtypes. TNFR1 predominates whereas TNFR2 activates transcription poorly. FEBS Lett. 2002;515:119. doi: 10.1016/s0014-5793(02)02450-x. [DOI] [PubMed] [Google Scholar]
- 44.Riches DW, Chan ED, Zahradka EA, Winston BW, Remigio LK, Lake FR. Cooperative signaling by tumor necrosis factor receptors CD120a (p55) and CD120b (p75) in the expression of nitric oxide and inducible nitric oxide synthase by mouse macrophages. J Biol Chem. 1998;273:22800. doi: 10.1074/jbc.273.35.22800. [DOI] [PubMed] [Google Scholar]
- 45.Calder CJ, Nicholson LB, Dick AD. A selective role for the TNF p55 receptor in autocrine signaling following IFN-gamma stimulation in experimental autoimmune uveoretinitis. J Immunol. 2005;175:6286. doi: 10.4049/jimmunol.175.10.6286. [DOI] [PubMed] [Google Scholar]
- 46.Masli S, Turpie B, Streilein JW. Thrombospondin orchestrates the tolerance-promoting properties of TGFbeta-treated antigen-presenting cells. Int Immunol. 2006;18:689. doi: 10.1093/intimm/dxl006. [DOI] [PubMed] [Google Scholar]