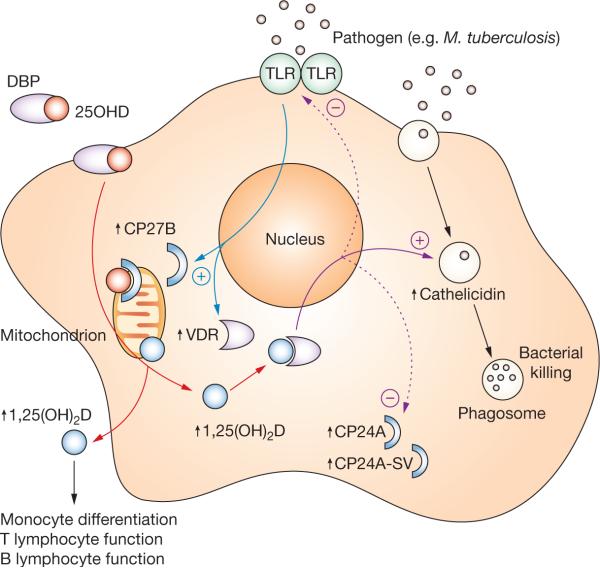
Figure 1.
Vitamin D and innate immunity. Activation of macrophage TLR (e.g. TLR2) signaling by pathogens such as as Mycobacterium tuberculosis results in the transcriptional induction of VDR and CP27B expression (blue arrows). Circulating 25OHD (red circles) bound to plasma DBP enters macrophages (red arrows) and is converted to 1,25(OH)2D (blue circles) by mitochondrial CP27B, and can bind to the VDR in the cell. Once bound to VDR, 1,25(OH)2D is able to act as a transcriptional factor leading to the induction of cathelicidin expression (solid purple arrow). Incorporation into phagosomes containing internalized pathogen enables cathelicidin to function as an antibacterial agent. As well as upregulating cathelicidin expression, macrophage synthesis of 1,25(OH)2D can also facilitate negative autoregulation (dashed purple arrows), firstly via increased expression of the feedback enzyme CP24A and its decoy CP24A-SV, and secondly via downregulation of TLR expression. In parallel with autocrine effects on innate antibacterial function, macrophage CP27B might also induce paracrine responses in monocytes, and T or B lymphocytes as a consequence of 1,25(OH)2D secretion. Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; CP24A, 1,25(OH)2D 24-hydroxylase; CP24A-SV, 1,25(OH)2D 24-hydroxylase splice variant; CP27B, 25OHD-1α hydroxylase; DBP, vitamin-D-binding protein; TLR, Toll-like receptor 2; VDR, vitamin D receptor.