Abstract
Increased adiposity is associated with insulin resistance (IR) and an inflammatory response in adults. We tested the hypotheses that cytokines associated with adiposity are also correlated with IR in early adolescents and that these relationships are moderated by weight status, levels of vigorous physical activity (VPA), or maximal aerobic power (pVO2max). Body mass, stature, and a fasting blood sample were obtained from 120 mid-pubertal adolescents (60 girls & 60 boys). Habitual VPA was obtained by a survey. Predicted VO2max was determined using a cycle-ergometer test. Weight status was based on body mass index percentiles (normal weight = BMI < 75th %tile and overweight = BMI > 95th %tile). Glucose, insulin, adiponectin, resistin, tumor necrosis factor-α, and interleukin-6 were measured, and IR index was based on the Homeostatic Model Assessment (HOMA). Adiponectin, resistin and TNF-α were associated with IR in all adolescents (R2=0.329, p<0.001, R2=0.152, p=0.001, R2=0.141, p=0.002; respectively), but IL-6 was not (R2=0.148, p=0.114). The degree of association between adiponectin and IR was stronger in overweight than in normal weight adolescents (p<0.050). When regression models included weight status, neither TNF-α nor resistin were significantly related to IR (p>0.050). Exercise did not moderate the association between these cytokines and IR. However, higher levels of VPA and/or pVO2max were associated with higher adiponectin, lower resistin and lower TNF- α in at least one of the genders. Our results indicate that the pathophysiology of obesity is already established in early adolescents. Increased adiposity, resulting in reduced adiponectin and increased resistin and TNF-α may link these cytokines with insulin resistance in adolescents.
1. Introduction
Increased adiposity has been strongly associated with insulin resistance in youth [1]. However, the link between adipose tissue and the increased resistance to insulin is still unclear [2-3]. Cytokines released by the adipose tissue, such as adiponectin, resistin, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), appear to link adiposity with insulin resistance in youth [4-8], as in adults [3, 10-15]. Adiponectin increases glucose uptake and free fatty acid oxidation in skeletal muscle [16] and is associated with decreased insulin resistance in youth and adults [4, 5, 8, 10]. The association between resistin and insulin resistance is equivocal [2, 17]. Studies in youth have not shown this association [18] and studies in adults presented contradictory findings [11, 19, 20]. TNF-α and IL-6 act to inhibit insulin signaling in the adipose tissue [12, 21]. Although TNF-α and IL-6 have been associated with insulin resistance in adults [13-15], there is little data in youth [6, 9, 15]. In pre-pubertal children there was no association between TNF-α and insulin resistance [15], while in another group of children soluble TNF-α receptor but not TNF-α was related to insulin resistance [6]. Similarly, IL-6 has been mostly related to adiposity in youth and with the metabolic syndrome [7, 9] but no clear link has been made between insulin resistance and this cytokine in youth [9].
During puberty there is an increase in insulin resistance [22] that may be related to changes in growth hormone, sex hormones and increases and redistribution of body fat [1]. All four of the previously mentioned cytokines have been associated with body fat indicators in youth [4, 5, 7-9, 18]. Therefore, it could be expected that the strength of the association between cytokines and insulin resistance is higher in overweight compared to normal weight youth. Similarly, decreased physical activity levels and maximal aerobic power have been associated with increased insulin resistance [23, 24]. Levels of these cytokines appear to respond favorably to chronic physical activity [25, 26] and exercise training [27-29] in youth and in adults. It is speculated that increased but not decreased physical activity or aerobic power, an indicator of cardiovascular fitness, might attenuate, if existent, the relationship between insulin resistance and resistin, TNF-α, and IL-6.
To our knowledge, this is the first study to explore associations between all four cytokines (adiponectin, resistin, IL-6 and TNF-α) and insulin resistance in male and female adolescents, and to examine the effect of weight status, physical activity levels, and aerobic power as moderators of this association. Additionally, this study included a multiethnic (mostly bi-racial) sample of adolescents in mid-puberty, the critical stage for a high degree of insulin resistance and changes in body fat content. This study tested two hypotheses: (1) cytokines (adiponectin, resistin, IL-6 and TNF-α) are associated with insulin resistance in mid-pubertal adolescents as previously shown in adults, and (2) overweight status would have opposite moderating effects than physical activity or aerobic power with regard to the associations between these cytokines and insulin resistance.
2. Subjects and Methods
2.1. Subjects
Participants from the Cardiovascular Health in Children and Youth Study (CHIC III) between the years 2000 and 2003 were the subjects for this cross-sectional study [30]. Sixty girls and sixty boys, ages 10 to 14 years, who were mid pubertal (Tanner stages 2-4), were randomly selected from sub-groups of 437 adolescents based on their weight status (normal weight versus overweight) and levels of habitual vigorous physical activity (VPA). Half of the adolescents selected were overweight (BMI>95th percentile using Centers for Disease Control and Prevention [CDC] norms), and half had a normal weight (BMI<75th percentile using CDC norms) [31]. Half of the adolescents also reported ≥180 min/week of habitual VPA (High-VPA) while the others reported ≤120 min/week of habitual VPA (Low-VPA). The participants signed an assent, and their parents or guardians signed an informed consent, approved by the Institutional Review Board, that indicated their agreement to the use of the stored blood sample for further analyses related to obesity.
2.2. Methods
Physical measures, habitual physical activity, pubertal status, a fasting blood sample, and predicted maximal aerobic power (pVO2max) were obtained in the school setting by trained and certified research assistants. Measurements of stature and body mass were completed within three days of blood sampling. The habitual physical activity survey and the pubertal development questionnaire were completed in small groups under the supervision of a trained research assistant.
All stature and body mass measurements were conducted with the subject dressed in shorts and t-shirt, without shoes and with pockets emptied. Stature was measured to the nearest 0.1 cm using a stadiometer (Perspective Enterprises, Kalamazoo, MI) and body mass was obtained to the nearest 0.1 kg using an electronic scale model 5602 (Scale-Tronix, Carol Stream, IL). Body mass index (BMI) was computed dividing body mass in kg by stature in meters squared (kg/m2). BMI was converted into a BMI percentile based on CDC norms for age and gender [31]. Body mass index percentile was used to classify adolescents as normal weight or overweight as previously indicated.
Pubertal developmental stage (Tanner stages 1-5) was assessed using a self-administered survey. The items assessing physical development included growth spurt in height, pubic hair and skin change (boys and girls), facial hair growth and voice change (boys), and breast development and menarche (girls) [32]. Pubertal developmental stage was estimated (1-5) based on a composite score derived from all the above mentioned survey items. This survey has been correlated against physician ratings (r=0.61- 0.67) as well as self-ratings from Tanner stage pictures (r=0.72- 0.80) [32].
Habitual VPA was obtained from a previously validated physical activity survey that had a test-retest correlation coefficient of r= 0.70 [33]. The survey asked the youth to check how often during a week they participated for at least 15 minutes in 32 activities common to North Carolina youth. Frequency answers ranged from never to daily (scores 0-6). The intensities of the 32 activities were determined using the Compendium of Physical Activity [34] and expressed in metabolic equivalents (METs). One MET represents the average resting oxygen consumption per kilogram body mass per min, e.g. 1 MET= 3.5 mL/kg/min. Only 9 of the activities included in the survey were of vigorous intensity (METs ≥ 6). The VPA score was calculated by summing the number of sessions per week for these 9 activities. Scores had a possible range from 0 to 54 sessions per week. Adolescents who reported more than 12 sessions per week of VPA were considered to have High-VPA, and those who reported less than 8 sessions per week were considered to have Low-VPA. This classification was based on the results of a larger study on 437 adolescents (including this subgroup of 120 adolescents) that showed that higher amounts of VPA were inversely related to insulin resistance, whereas neither total habitual physical activity, nor moderate intensity activities (METs ≤ 3.8) were associated with improved insulin resistance[24]. Peak aerobic power (pVO2max), an indicator of cardiovascular fitness, was predicted from the previously validated PWC195 cycle ergometry test which has been highly correlated (r=0.807) with maximal aerobic power [35].
Blood samples were obtained between 7-8 am after an overnight fast. Non-compliant participants were not included. Samples were collected in EDTA-containing tubes and immediately centrifuged at 4°C to obtain plasma. Plasma samples were kept on dry-ice during transportation from the testing sites and were stored at −80°C until analyzed. Glucose was determined using the hexokinase oxidase method which has a sensitivity of 1mg/dL (0.05551 mmol/L). Plasma insulin concentrations were determined by Linco Laboratories (St. Charles, MO) with a coefficient of variation (CV) of 8.0%. Plasma adiponectin, resistin, TNF-α, and IL-6 were obtained from enzyme immunoassay procedures. Adiponectin and resistin were assayed utilizing kits from Linco Research Inc. (St.Charles, MO). The adiponectin assay had a limit of sensitivity of 0.78 ng/mL with intra and inter-assay CVs of <10% and 11.6%, respectively. The resistin assay had a sensitivity of 0.5 ng/mL, with intra and inter-assay CVs of <10% and 11.2%, respectively. TNF-α and IL-6 were assessed utilizing kits from R & D Systems (Minneapolis, MN). The TNF-α kit had a sensitivity of 0.5 pg/ml, while the IL-6 kit had a sensitivity of 0.2 pg/ml. The intra-assay CV for TNF-α and IL- 6 assays was <10%. The inter-assay CV for TNF-α was 12.2% and for IL- 6 was 18.2 %. Insulin resistance was assessed using the Homeostatic Model Assessment for insulin resistance (HOMA) [36].HOMA has been validated in children and adolescents [37] and was computed as follows: HOMA= insulin [μIU/mL] × glucose [mmol/L]/22.5.
2.3. Statistical analysis
Mean and standard deviation values were computed for all variables for all subjects in the study. Frequency tables were generated to determine the number of Caucasian, African American and “other” youth. Other ethnicities included: Asian, Native and other. Separate analyses of variance were conducted for the physical characteristics, metabolic factors, and exercise variables to determine differences between the genders and ethnic groups. A Mann-Whitney test was used to determine differences in pubertal status between the genders and between African American and Caucasian youth.
Cytokines, insulin, glucose and HOMA values were transformed using the natural logarithm for all the regression analyses, because these variables were not normally distributed. Exploratory Pearson product correlations were computed to determine associations among HOMA, cytokines, BMI, and pVO2max. Because VPA was dichotomized into two groups, Spearman Rho correlations were computed between this variable, HOMA, and the cytokines.
To determine if the cytokines were associated with insulin resistance, five multiple regression models were computed for each cytokine using the “enter” method of regression analysis. This method evaluates the significance of the relationship of every variable in the model when entered all together. The first model included only the cytokine controlling for gender and ethnicity. The second, third, and fourth models tested the moderator effect of weight status, VPA, and pVO2max respectively. Thus, the second model tested if the relationship between the cytokine and insulin resistance was different depending on the weight status (normal weight vs. overweight). The third model tested if the relationship between the cytokine and insulin resistance was different depending on the levels of VPA (High vs. Low). The fourth model tested if the relationship between the cytokine and insulin resistance was different depending on the levels of VO2max. Significant parameters from models 2-4 were used to test a final model. The interaction terms (cytokine * weight status, cytokine * VPA, and cytokine * VO2max) were included because weight status, VPA, and pVO2max may moderate the association between the cytokines and insulin resistance [11, 7-8, 25-29]. Statistical significance was set at p<0.05. Analyses were conducted utilizing Statistical Package for the Social Sciences (SPSS) version 9.0 for Windows (Chicago, IL).
3. Results
The characteristics of the subjects are presented in Table 1. Racial distribution was 57% African-American, 37% Caucasian, and 6% other ethnic groups. There were 60 girls and 60 boys participating in this study. The distribution of normal and overweight adolescents in the sample was the same, and the number of adolescents reporting high VPA or low VPA was equal.
TABLE 1.
Physical and metabolic characteristics of subjects by ethnic groups (African-American, Caucasian and Other) and gender (girls, boys): mean ± standard deviation or frequencies (n)
| African-American | Caucasian | Other | ||||
|---|---|---|---|---|---|---|
| Girls (n=34) |
Boys (n=35) |
Girls (n=23) |
Boys (n=21) |
Girls (n=3) |
Boys (n=4) |
|
| Demographic and physical characteristics | ||||||
| Age (years) | 11.6 ±0.7 | 11.9 ±0.7 | 11.9 ± 0.9 | 12.1 ±0.9 | 12.3 ± 0.6 | 12.3 ±1.0 |
| Pubertal status | ||||||
| Stage 2/ Stage 3/ Stage 4* | 4/11/19 | 9/23/3 | 7/8/8 | 7/10/4 | 1/1/1 | 1/0/3 |
| Weight status | ||||||
| Normal/Overweight | 13/21 | 17/18 | 16/7 | 10/11 | 1/2 | 3/1 |
| BMI (kg/m2) † | 26.0 ± 8.2 | 23.8 ± 7.7 | 20.6 ± 4.6 | 22.9 ± 6.1 | 24.9 ± 8.6 | 19.4 ± 5.0 |
| VPA (sessions/week) | 9.5 ± 7.4 | 15.1 ± 8.2 | 10.0 ± 8.9 | 5.8 ± 6.75 | 13.7 ± 9.5 | 13.5 ± 9.5 |
| pVO2max (ml/kg/min)* | 32.9 ± 11.2 | 39.6 ± 11.7 | 34.8 ± 8.6 | 35.9 ± 12.1 | 33.6 ± 7.7 | 40.0 ± 2.8 |
| Metabolic factors | ||||||
| TNF- α (pg/ml) | 1.49 ± 1.3 | 1.84 ± 1.31 | 1.43 ± 0.8 | 1.46 ± 0.60 | 2.71 ± 1.82 | 1.92 ± 0.82 |
| IL-6 (pg/ml) | 1.70 ± 1.6 | 1.74 ± 2.03 | 1.56 ± 1.5 | 1.14 ± 1.41 | 3.32 ± 5.14 | 1.83 ± 2.16 |
| Resistin (ng/ml) † | 10.2 ± 6.3 | 10.5 ± 4.8 | 12.1 ± 4.5 | 11.8 ± 4.5 | 7.1 ± 0.9 | 8.0 ± 1.6 |
| Adiponectin (ng/ml) † | 9.6 ± 5.8 | 9.1 ± 4.2 | 11.6 ± 5.3 | 10.8 ± 4.4 | 10.9 ± 3.9 | 8.6 ± 0.2 |
| Glucose (mmol/L) | 4.9 ± 0.4 | 5.0±0.3 | 4.9 ± 0.4 | 4.9 ± 0.3 | 5.0 ± 0.4 | 5.0 ± 0.3 |
| Insulin (pmol/L) | 189.5±129.0 | 127.5±121.1 | 85.5 ± 43.2 | 100.4 ± 63.6 | 138.7 ± 83.2 | 62.8 ± 39.9 |
| HOMA | 5.8 ± 4.0 | 4.0± 4.0 | 2.60 ± 1.4 | 3.0 ± 2.0 | 4.4 ± 2.8 | 1.9 ± 1.3 |
p < 0.05 African American vs. Caucasian
p < 0.05 Girls vs. Boys
African American youth were heavier and had higher BMI than Caucasian youth (p<0.050). African American youth also had lower adiponectin (p=0.029), higher resistin concentrations (p=0.029), and higher HOMA (p=0.002) than Caucasian youth. There were no differences in puberty between both groups (p=0.226) Lastly, African American youth had higher VPA scores than Caucasian (p=0.007). There were no statistically significant group differences between the genders for age, race, stature, body mass, BMI, cytokines, HOMA, or VPA (p>0.050 for all). The girls were at higher mean pubertal stage than the boys (p<0.050) and the boys had higher pVO2max than the girls (p<0.050).
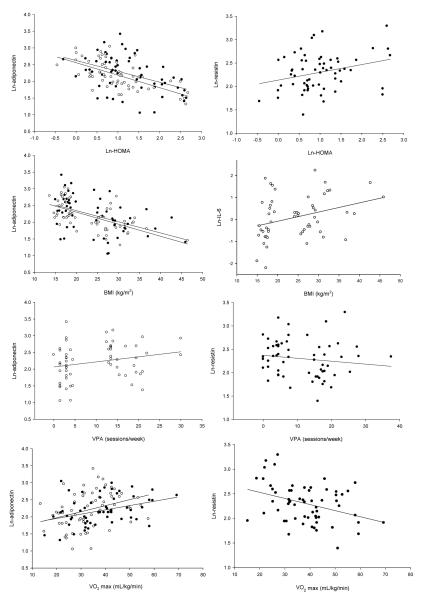
The univariate correlations indicated that HOMA was significantly negatively associated with adiponectin in the boys and in the girls (r=-0.628 and r=-0.438, p<0.008, respectively) and significantly positively associated with resistin in the boys only (r=0.291, p=0.024). HOMA was not associated with either TNF-α, or IL-6 in either gender. Body mass index was associated with adiponectin in both girls and boys (r=-0.469, r=-0.610; p<0.001, respectively), and with IL-6 in the girls (r=0.329; p=0.01) but not the boys. Positive trends were obtained between BMI and resistin (r=0.201), or IL-6 (r=0.244) in boys. VPA was associated only with adiponectin in the girls (r=0.306, p=0.017), and with resistin in the boys (r=-0.286, p=0.027). Peak VO2max was associated with adiponectin in the girls and in the boys (r=0.337, r=0.351; p<0.05, respectively), with resistin in the boys (r=-0.374; p<0.05), and with TNF- α in the girls (r=-0.314; p<0.05) (Fig. 1).
Figure 1.
Scatterplots presenting significant associations between cytokines, HOMA, BMI, and exercise. Filled circles are used for girls’ data and open circles are used for boys’ data.
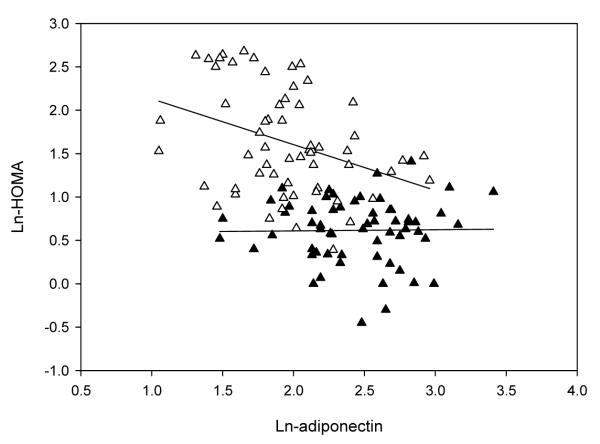
Table 2 presents select results of the five multiple regression models in which HOMA was regressed separately with each cytokine (adiponectin, resistin, TNF-α, or IL-6). Only model 1, significant models that contained interaction terms, and the final models are presented in the Table. Adiponectin was negatively associated with HOMA (Model 1, p<0.001 for β-coefficient) and weight status significantly moderated the relationship between adiponectin and HOMA (Model 2, p=0.047 for β-coefficient) (Fig. 2). Neither VPA nor pVO2max moderated the relationship between adiponectin and HOMA (Models 3 and 4, p>0.05 for interaction β-coefficients) but pVO2max was a significant predictor of HOMA (Model 4 p=0.036 for β-coefficient). Moreover, when pVO2max was included in the final model, weight status did not moderate the relationship between adiponectin and HOMA (Final Model p=0.080 for interaction β-coefficient)
TABLE 2.
Multivariate regression models for the relationship between insulin resistance (HOMA-IR) and cytokines, weight status, VPA, and VO2max, controlling for gender and ethnicity
| Model | Independent variable | β-coefficient | SE | P | Model R2 | P |
|---|---|---|---|---|---|---|
| 1 | Adiponectin | -0.711 | 0.118 | 0.000 | 0.329 | 0.000 |
| 2 | Adiponectin | -0.021 | 0.151 | 0.892 | ||
| Weight status | 1.815 | 0.486 | 0.000 | |||
| Adiponectin*weight status | -0.431 | 0.215 | 0.047 | 0.605 | 0.000 | |
| Final | Adiponectin | -0.052 | 0.149 | 0.726 | ||
| Weight status | 1.513 | 0.500 | 0.003 | |||
| Adiponectin*weight status | -0.377 | 0.213 | 0.080 | |||
| pVO2max | -0.012 | 0.006 | 0.037 | 0.620 | 0.000 | |
| 1 | Resistin | 0.329 | 0.159 | 0.041 | 0.152 | 0.001 |
| 3 | Resistin | -0.116 | 0.251 | 0.646 | ||
| VPA | -1.817 | 0.732 | 0.015 | |||
| VPA*resistin | 0.708 | 0.317 | 0.028 | 0.207 | 0.000 | |
| Final | Resistin | -0.191 | 0.176 | 0.279 | ||
| Weight status | 0.788 | 0.120 | 0.000 | |||
| VPA | -1.337 | 0.509 | 0.010 | |||
| VPA*resistin | 0.518 | 0.220 | 0.020 | |||
| pVO2max | -0.010 | 0.006 | 0.077 | 0.627 | 0.000 | |
| 1 | TNF- α | 0.223 | 0.100 | 0.027 | 0.141 | 0.002 |
| Final | TNF-α | 0.032 | 0.073 | 0.661 | ||
| Weight status | 0.765 | 0.130 | 0.000 | |||
| pVO2 max | -0.012 | 0.006 | 0.048 | 0.580 | 0.000 | |
| 1 | IL-6 | 0.172 | 0.071 | 0.114 | 0.148 | 0.001 |
Weight status coding: Normal=0 (BMI <75th %tile); Overweight=1 (BMI >95th %tile) Vigorous physical activity (VPA) coding: High=1; Low=0. Gender coding: Girls=1; Boys=0. Gender and ethnicity β-coefficients not presented. All cytokines were logarithmically transformed. Each IR and cytokine final model presented in this table includes only significant parameters tested in Models 1-4.
Model 1: HOMA-IR = cytokine
Model 2: HOMA-IR = cytokine + weight status + cytokine*weight status
Model 3: HOMA-IR = cytokine + VPA + cytokine*VPA
Model 4: HOMA-IR = cytokine + pVO2 max + cytokine* pVO2 max
Figure 2.
Scatterplot presenting the moderatoreffect of weight status (normal weight vs. overweight) in the association between adiponectin and insulin resistance. Open triangles are used for overweight adolescents’ data, and filled triangles are used for normal weight adolescents’ data.
Resistin was weakly associated with HOMA (Model 1, R2= 0.152; p=0.001). Weight status did not interact between HOMA and resistin (Model 2, p>0.05 for β-coefficient) but was a significant predictor of HOMA (p=0.006 for β-coefficient). VPA moderated the association between resistin and HOMA (Model 3, p=0.028 for β-coefficient). Predicted VO2 max did not interact between resistin and HOMA (Model 4, p>0.05 for β-coefficient), but was significantly associated with HOMA (p=0.010). In the final model, weight status was associated with HOMA, and VPA interacted in the association between resistin and HOMA (p<0.05 for all).
TNF-α was weakly associated with HOMA (R2= 0.141; p=0.002). Although weight status did not directly moderate the association between TNF-α and HOMA (Model 2, p=0.233 for interaction β-coefficient) when included in the model, the association between TNF-α and HOMA was no longer significant (p=0.231 for TNF- α β-coefficient). Neither VPA nor pVO2max interacted in the association between TNF-α and HOMA (Models 3 and 4, p>0.05 for both interactions β-coefficients), but pVO2max was a significant predictor of HOMA (Model 4, p<0.001 for β-coefficient). Interleukin-6 was not a significant predictor of HOMA (p=0.114 for β-coefficient).
4. Discussion
Insulin resistance, cytokines and weight status
We chose to examine the association between selected cytokines and insulin resistance in adolescents. The selected four cytokines were studied because of existing data in adults suggesting such associations existed [10, 11, 12-14]. Although many studies have demonstrated a negative association between adiponectin and insulin resistance [4-5, 9], we hypothesized a different association between adiponectin and insulin resistance depending on weight status. We showed that in overweight adolescents, an increase of log-adiponectin by 1 unit predicted a 45.1% decrease in the log HOMA. However, in the normal weight adolescents, a 1 unit change moderated the HOMA by 2 %. This finding suggests that the protective role of adiponectin on insulin resistance is more relevant in overweight youth. Weiss et al. showed a similar relationship in moderately obese adolescents, but not in severely obese adolescents [38]. We speculate that in normal weight adolescents, the concentrations of adiponectin, other cytokines, triglycerides, and leptin are normal, and therefore, the protective role of adiponectin is minimal. Once adiposity levels increase further, as in severely obese youth, adiponectin’s protective role might be superseded by the other mentioned adipokines. The moderator effect of weight status in this association was also not relevant when aerobic power was included in the model; perhaps because of the inclusion of body mass in aerobic power units.
One of our novel findings was that resistin was related to HOMA in our adolescent population. In adults, Silha et al. [11] have shown similar results to ours; yet other studies have failed to show this association [18, 19]. In contrast to our results, Gerber and colleagues failed to show a relationship between insulin resistance indicators and resistin in youth, possibly because of their study’s low statistical power [18]. Our results suggest that insulin resistance is weakly associated with resistin; however this association may be dependent on the association between adiposity and resistin, as shown in adults [20].
Contrasting the results obtained in children [6-8, 15] TNF-α, was associated with HOMA in the adolescents of the present study. Others have shown an association between soluble TNF-α receptor 2 and insulin resistance, but not TNF-α [6]. Our results should be interpreted with caution, since the regression model only explained 14.1% of the variance in HOMA. Moreover, when weight status was considered, TNF-α did not explain a significant variance in HOMA. The importance of TNF-α in the development of insulin resistance may become more evident as youth age [15], as different factors contribute to the development of this insulin resistant state [39].
We found no association between IL-6 concentrations and HOMA regardless of weight status. Similarly, Nemet et al. showed no correlation between IL-6 and fasting insulin [9]. Weiss et al. showed no differences in the concentrations of IL-6 across categories of insulin resistance in a large sample of obese children and adolescents [38]; though Kelly and associates presented a positive trend in IL-6 in youth with metabolic syndrome [7]. The development of insulin resistance is multifactorial in origin; possibly, IL-6 becomes a more important player as adolescents age, because its effects may become additive to the effects of other increased cytokines, such as TNF-α, Interleukin-1β and leptin [39]. The combination of elevated inflammatory and atherogenic cytokines present in youth with metabolic syndrome [7] would support this previous speculation.
Exercise as a moderator of the relationship between IR and cytokines
In the resistin model, HOMA was negatively associated with habitual VPA suggesting that more sessions per week of VPA are associated with lower insulin resistance. However, VPA interacted in the association between resistin and HOMA in the opposite way of what we anticipated. Specifically, a 1 unit increase in the natural log of resistin predicted a 59.2% increase in the natural log of HOMA in those adolescents who had high VPA. In contrast, an increase of 1 unit in the natural log of resistin predicted an 11 % decrease in the natural log of HOMA in those adolescents who had low VPA. Neither weight status, overall adiposity as indicated by BMI, puberty, nor ethnicity explained this association. Therefore, further research is necessary to explore the effect of exercise in the relationship between resistin and insulin resistance.
In contrast with what we hypothesized, neither habitual VPA nor pVO2max moderated the association between insulin resistance and adiponectin or TNF-α. Although pVO2max was associated with HOMA in most of the regression models, pVO2max was not significant when weight status was included in the model for resistin. This could be because of the inclusion of body mass in the units of aerobic power as well as in BMI (kg/m2). Findings in adults [26, 27, 28] led us to hypothesize that the inclusion of exercise in our models would strengthen the association between adiponectin and HOMA, and would weaken all the other associations. In obese youth, increasing the levels of physical activity resulted in decreased insulin resistance and IL-6 concentrations [29]. Supporting our speculations, a recently published study in adolescents showed increased circulating adiponectin, and decreased TNF- α in physically active versus sedentary girls [25]. In the present study we show that increased sessions perweek of physical activity in adolescence can be associated with higher adiponectin concentrations in the girls and lower resistin concentrations in the boys. In contrast to Nemet et al. [9] aerobic power was negatively associated with lower TNF- α. The difference in the findings between Nemet et al. and ours can be explained by the different units used to express aerobic power (mL/min vs. mL/kg/min).
Our findings mirrored previous results related to adiponectin and insulin resistance [6, 8, 17] and expanded previous studies’ findings by including other cytokines of interest and other relationships. However, our findings present some methodological limitations. VPA was self-reported and there could be recall bias in this measurement. We used the Compendium of Physical Activities to determine the intensity of physical activity as others have done [25] but this compendium is based on data from adults [34]. The use of a more sensitive measure of insulin resistance might have strengthened the relationships between the cytokines and insulin resistance. However, the HOMA (derived from a mathematical model) has been shown to be an adequate indicator of insulin resistance in youth [37] and to be associated with VPA and pVO2max [24]. Lastly, cytokines as well as hormones can be acutely modified by previous physical activity or exercise [40] limiting our cytokine results.
Conclusion
Our results show that, in adolescents, insulin resistance is related to decreased adiponectin, increased resistin and TNF-α. The link between these cytokines and insulin resistance is probably related to increased adiposity mostly in overweight youth. Habitual VPA and pVO2max do not appear to have a strong influence on the relationships between these cytokines and insulin resistance in either normal or overweight youth. But this is not to say that higher amounts of physical activity [23-26] or exercise interventions [27-29] would not improve insulin resistance or these cytokines. Moreover, our results show that higher levels of habitual VPA and pVO2max are correlated with increased adiponectin and decreased resistin, both somewhat protective against insulin resistance. Similarly, the present results support the negative association between insulin resistance and increased aerobic power and physical activity previously shown [23, 24].
Although our findings present methodological and statistical limitations as the degree of the associations is moderate to low, they suggest that increased levels of adiposity, resulting in decreased adiponectin, increased resistin and TNF-α concentrations, may link being overweight with insulin resistance during adolescence. Thus, the inflammatory condition that appears to link obesity with insulin resistance in adulthood [2, 3] is also present during puberty. The efforts to reduce insulin resistance in adolescents should focus on body fat mass loss and prevention of body fat mass gain through increased physical activity, improved aerobic power, and a healthy diet.
Acknowledgements
This study was supported by the Graduate Student Trust Fund UNC-Chapel Hill (to D.A.R.), the Department of Exercise and Sport Science, UNC-Chapel Hill (to D.A.R.), NIH-NINR-RO1-1837 (to J.S.H.), and 1K23-RR-021979 (to A.M.H.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Roemmich JN, Clark P, Lusk M, Friel A. Pubertal alterations in growth and body composition. Pubertal insulin resistance: relationship to adiposity, body fat distribution and hormone release. Int J Obes. 2002;26:701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- [2].Smith S, Ravussin E. Emerging paradigms for understanding fatness and diabetes risk. Curr Diab Rep. 2002;2:223–230. doi: 10.1007/s11892-002-0087-1. [DOI] [PubMed] [Google Scholar]
- [3].Fernandez-Real J, Ricart W. Insulin Resistance and Chronic Cardiovascular Inflammatory Syndrome. Endocrine Reviews. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- [4].Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, et al. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- [5].Huang K, Lue B, Yen R, Shen C, Ho S, Tai T, Yang W. Plasma adiponectin levels and metabolic factors in non-diabetic adolescents. Obes Res. 2004;12:119–224. doi: 10.1038/oby.2004.16. [DOI] [PubMed] [Google Scholar]
- [6].Gupta A, Ten S, Anhalt H. Serum levels of soluble necrosis factor-alpha receptor 2 are linked to insulin resistance and glucose tolerance in children. J Pediatr Endocrinol Metab. 2005;18:75–82. doi: 10.1515/jpem.2005.18.1.75. [DOI] [PubMed] [Google Scholar]
- [7].Kelly AS, Steinberg J, Kaiser DR, Olson TP, Bank AJ, Dengel DR. Oxidative stress and adverse adipokine profile characterize the metabolic syndrome in children. J Cardiometab Syndr. 2006;1:248–252. doi: 10.1111/j.1559-4564.2006.05758.x. [DOI] [PubMed] [Google Scholar]
- [8].Valle M, Martos R, Gascón F, Cañete R, Zafra MA, Morales R, Zafra MA, Morales R. Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab. 2005;31:55–62. doi: 10.1016/s1262-3636(07)70167-2. [DOI] [PubMed] [Google Scholar]
- [9].Nemet D, Wang P, Funahashi T, Matsuzawa Y, Tanaka S, Engelman L, Cooper D. Cytokines, body composition and fitness in children. Pediatr Res. 2003;53:148–152. doi: 10.1203/00006450-200301000-00025. [DOI] [PubMed] [Google Scholar]
- [10].Jansson P, Pellme F, Hammarstedt A, Sandqvist M. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. FASEB J. 2003;17:1434–1440. doi: 10.1096/fj.02-1132com. [DOI] [PubMed] [Google Scholar]
- [11].Silha J, Krsek M, Skrha JV, Sucharda P, Nyomba B, Murphy L. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003;149:331–335. doi: 10.1530/eje.0.1490331. [DOI] [PubMed] [Google Scholar]
- [12].Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) reduces gene and protein expression of IRS-1 and GLUT-4 and is overexpressed in human fat cells from insulin resistant subjects. Diabetol. 2003;46:1594–1603. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- [13].Fernandez-Real J, Broch M, Ricart W, Casamitjana R, Gutierrez C, Vendrell J, Richart C. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes. 1998;47:1757–1762. doi: 10.2337/diabetes.47.11.1757. [DOI] [PubMed] [Google Scholar]
- [14].Zinman B, Hanley A, Harris S, Kwan J, Fantus I. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1998;84:272–278. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- [15].Ijzerman RG, Voordouw JJ, Van Weissenbrunch MM, Yudkin JS, Serné EH, Delemarre-van de Wal HA, Stehouwer CD. TNF-alpha levels are associated with skin capillary recruitment in humans: a potential explanation for the relationship between TNF-alpha and insulin resistance. Clin Sci. 2006;110:361–368. doi: 10.1042/CS20050314. [DOI] [PubMed] [Google Scholar]
- [16].Yamauchi T, Kamon J, Minokoshi Y, Ito Y. Adiponectin stimulates glucose utilization and fatty acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1–8. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- [17].Steppan C, Balley S, Baht S, Brown E. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- [18].Gerber M, Boettner A, Seidel B, Lammert A, Bar J, Schuster E, et al. Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab. 2005;90:4503–4509. doi: 10.1210/jc.2005-0437. [DOI] [PubMed] [Google Scholar]
- [19].Lee J, Chan J, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin resistant and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- [20].Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452–5455. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- [21].Liu L, Spekellen M, Rohrig K. Tumor necrosis factor-alpha acutely inhibits insulin signaling in human adipocytes. Diabetes. 1998;47:515–522. doi: 10.2337/diabetes.47.4.515. [DOI] [PubMed] [Google Scholar]
- [22].Potau N, Ibañez L, Riqué S, Carrascosa A. Pubertal changes in insulin secretion and peripheral insulin sensitivity. Horm Res. 1997;48:219–226. doi: 10.1159/000185519. [DOI] [PubMed] [Google Scholar]
- [23].Schmitz KH, Jacobs DR, Hong CP, Steinberg J, Moran A, Sinaiko AR. Association of physical activity with insulin sensitivity in children. Int J Obes. 2002;26:1310–1316. doi: 10.1038/sj.ijo.0802137. [DOI] [PubMed] [Google Scholar]
- [24].Rubin DA, McMurray RG, Harrell JS. Insulin resistance and weight status in adolescents: independent effects of intensity of physical activity and peak aerobic power. Ped Ex Sci. doi: 10.1123/pes.20.1.29. In press. [DOI] [PubMed] [Google Scholar]
- [25].Ischander M, Zaldivar F, Eliakim A, Nussbaum E, Dunton E, Leu S, Cooper DM, Schneider M. Physical activity, growth, and inflammatory mediators in BMI-matched female adolescents. Med Sci Sports Exerc. 2007;39:1131–1138. doi: 10.1249/mss.0b013e318053e7a2. [DOI] [PubMed] [Google Scholar]
- [26].Pischon T, Hankinson S, Hotamisligil S, Rifai N, Rimm E. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–1064. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- [27].Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- [28].Straczkowski M, Kowalska I, Dzienis-Straczkowska S, Stepieri A, Skibinska E, Szelachowska M, Kinalska I. Changes in tumor necrosis factor-a system and insulin sensitivity during an exercise training program in obese women with normal and impaired glucose tolerance. Eur J Endocrinol. 2001;145:273–280. doi: 10.1530/eje.0.1450273. [DOI] [PubMed] [Google Scholar]
- [29].Balagopal P, George D, Patton N, Yarandi H, Roberts W, Bayne E, Gidding S. Lifestyle-only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. J Pediatr. 2005;146:342–348. doi: 10.1016/j.jpeds.2004.11.033. [DOI] [PubMed] [Google Scholar]
- [30].McMurray RG, Harrell JS, Bangdiwala SI, Bradley CB, Deng S, Levine A. A school-based intervention can reduce body fat and blood pressure in young adolescents. J Adolescent Health. 2002;31:125–132. doi: 10.1016/s1054-139x(02)00348-8. [DOI] [PubMed] [Google Scholar]
- [31].Centers for Disease Control and Prevention. National Center for Health Statistics CDC growth charts: United States. 2000 May 30; http://www.cdc.gov/growthcharts/
- [32].Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adol. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- [33].Gilmer M, Speck B, Bradley CB, Harrell JS, Belyea M. The youth health survey: reliability and validity of an instrument for assessing cardiovascular health habits in adolescents. J School Health. 1996;66:106–111. [PubMed] [Google Scholar]
- [34].Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ. Compendium of Physical Activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- [35].McMurray RG, Guion WK, Ainsworth BE, Harrell JS. Predicting aerobic power in children. A comparison of two methods. J Sports Med Phys Fitness. 1998;38:227–33. [PubMed] [Google Scholar]
- [36].Turner R, Holman R, Matthews D, Hockaday TD, Peto J. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal insulin and glucose concentrations. Metabolism. 1979;28:1086–1096. doi: 10.1016/0026-0495(79)90146-x. [DOI] [PubMed] [Google Scholar]
- [37].Guzzaloni G, Grugni G, Mazzilli G, Moro D, Morabito F. Comparison between B-cell function and insulin resistance indexes in prepubertal and pubertal obese children. Metabolism. 2002;51:1011–1016. doi: 10.1053/meta.2002.34029. [DOI] [PubMed] [Google Scholar]
- [38].Weiss R, Dziura J, Burgert T, Tamborlane W, Taksali S, Yeckel C, Allen K. Obesity and metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- [39].Rhodes CJ. Type 2 diabetes —a matter of β-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- [40].Nemet D, Oh Y, Kim H, Hill M, Cooper D. Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics. 2002;110:681–689. doi: 10.1542/peds.110.4.681. [DOI] [PubMed] [Google Scholar]