Figure 1. Structure and gating models of the GlpG rhomboid protease from Escherichia coli.
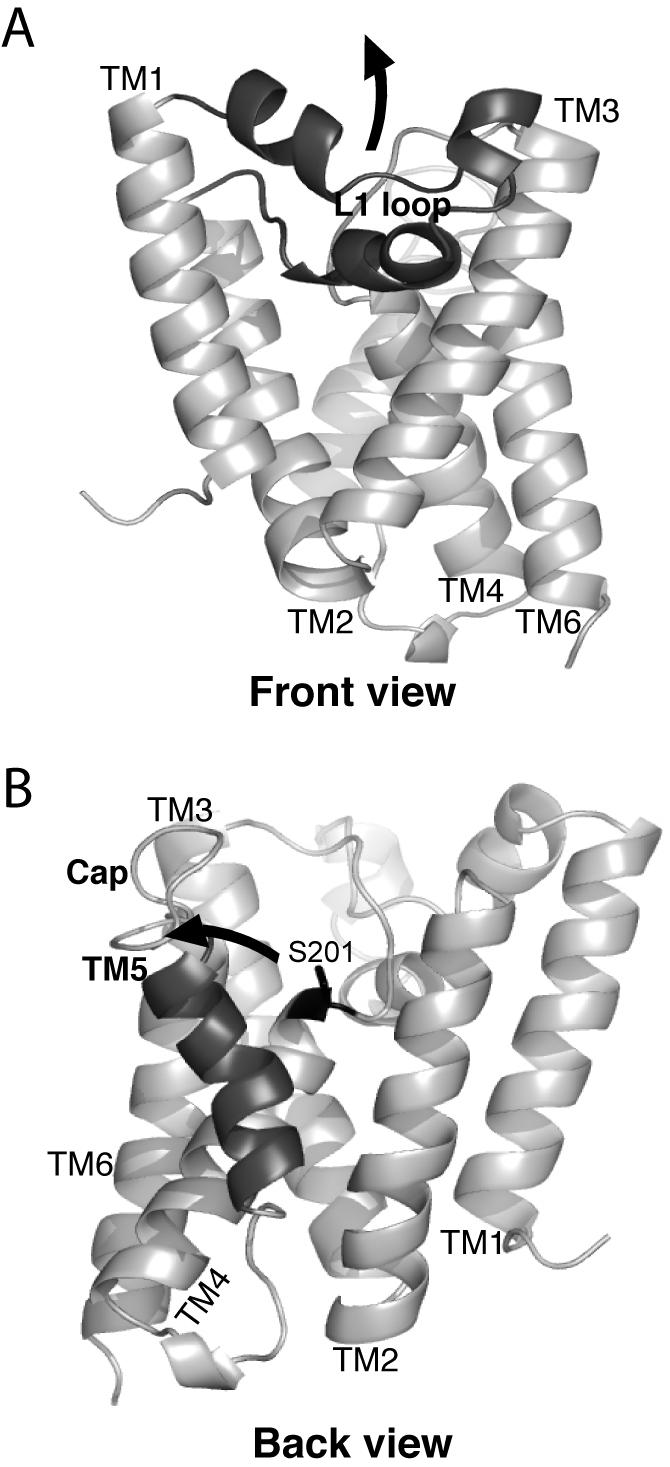
A. The ‘front view’ of the core domain of GlpG is shown laterally from the plane of the membrane, with the extracellular side up and the cytosolic side down. The membrane-submerged L1 loop is highlighted in black, and the hypothetical conformation change to allow substrate access between transmembrane helices 1 and 3 is depicted by an upward arrow. B. The ‘back view’ of the GlpG protease is shown laterally as above, with the transmembrane helix 5 highlighted in black. The open conformation is shown (coordinates from PDB N2RF), with an arrow depicting the proposed transmembrane segment 5 tilting and Cap movement that gates substrate access to the active site serine (in black ball-and-stick).
