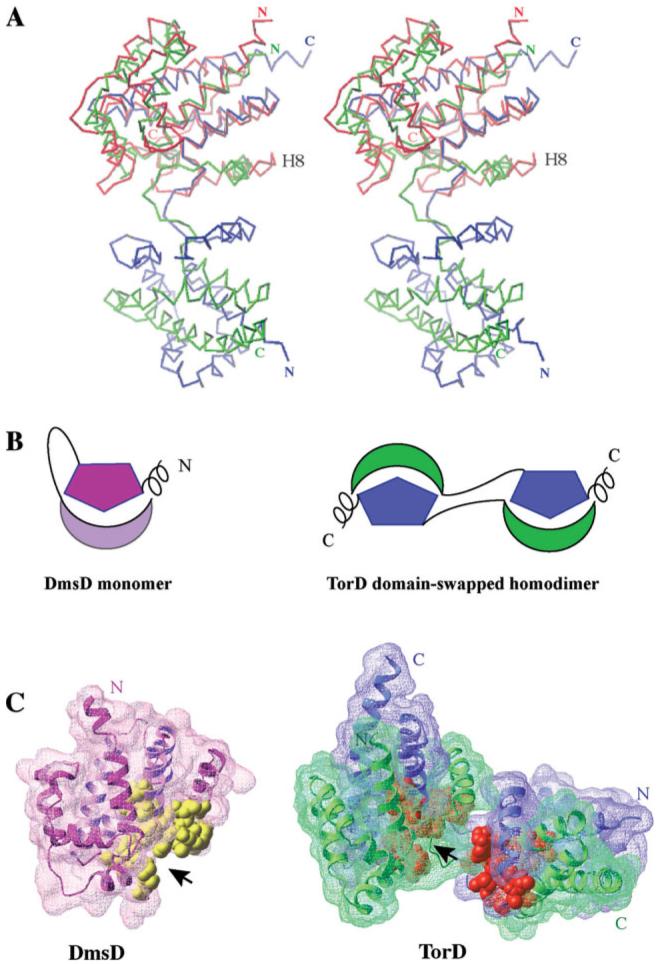
Figure 3.
(A) Stereo view of the DmsD structure (colored in red) superposed on one of the subunit of TorD homodimer. The two subunits of TorD are colored in blue and green. The most notable difference between the two structures is the orientation of the helix located before the long loop/hinge region, H8 in DmsD. (B) Cartoon representations of the DmsD monomer and the domain-swapped TorD homodimer. The helical extensions are located at the N-terminus of DmsD and the C-terminus of TorD. The hinge/loop regions between N-terminal moieties and C-terminal moieties are shown by lines. (C) Surface representations of DmsD monomer and TorD dimer with the conserved residues of TorD/DmsD family (see Fig. 2) displayed by CPK representation in yellow (DmsD) and gold (TorD), respectively. The two chains of TorD homodimer are colored in blue and green.