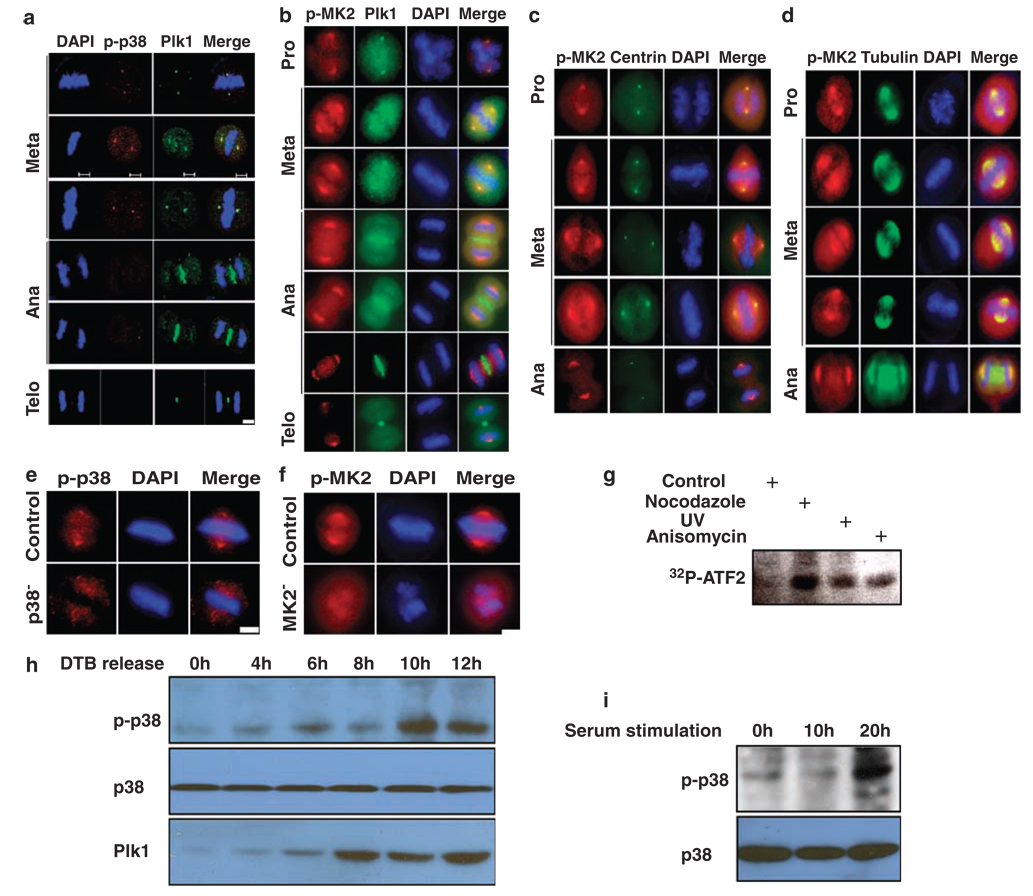
Figure 4.
Activation of the p38 MAP kinase pathway during mitosis. (a) Colocalization of Plk1 and phospho-p38α at the spindle poles during mitosis. HeLa cells were synchronized with the double thymidine block, released for 10 h and co-stained with Plk1 and phospho-p38α. (b) Cells were prepared as in (a), and co-stained with Plk1 and phospho-MK2. (c, d) HeLa cells stably expressing GFP-centrin (c) or GFP-tubulin (d) were prepared as in (a), and stained with phospho-MK2. Scale bar, 5 µm. (e, f) Spindle localization of phospho-p38α (p-p38)/MK2 is specific. HeLa cells were p38α- or MK2-depleted using RNA interference (RNAi) as in Figure 2a and stained with phospho-p38α (e) or phospho-MK2 (p-MK2) (f) antibody. Scale bar, 5 µm. (g) HeLa cells were treated with 200 ng/ml nocodazole for 12 h, 50 J/m2 UV irradiation or 50 ng/ml anisomycin for 4 h and harvested. Lysates were subjected to anti-p38α immunoprecipitation (IP)/kinase assays using purified GST-ATF2 as a substrate. (h) HeLa cells were synchronized with the double thymidine block protocol, released for different times and harvested for western blotting. (i) NIH3T3 cells were serum starved for 2 days, released into cell cycle upon 15% serum stimulation for different times and harvested for phospho-p38 western blot.