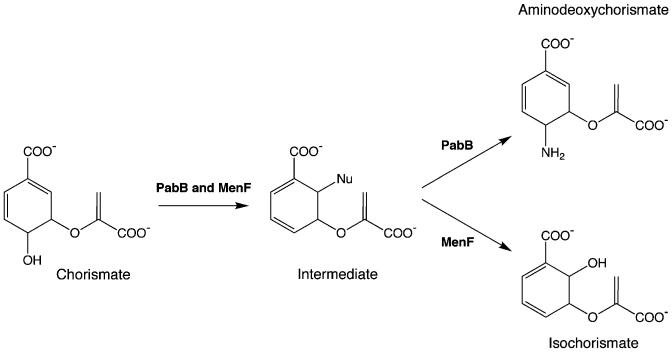
Fig. 2.
Catalytic promiscuity among chorismate-utilizing enzymes. PabB catalyzes the formation of 4-amino-4-deoxychorismate; the ΔpabB strain can be rescued by expression of its homologue, MenF (isochorismate synthase). The 2 enzymes act via a common intermediate, generated in an SN2′ mechanism that leads to addition of a nucleophile at C2, with concomitant elimination of the C4 hydroxyl group (He et al. 2004). In PabB, the nucleophile is the ∊-amino group of an active site lysine; the result is a covalent intermediate. In MenF, the nucleophile is assumed to be water, although its isozyme, EntC, can also utilize ammonia. Our results suggest that product release by MenF is sufficiently slow that ammonia can enter the active site and effect a second SN2′ displacement, yielding aminodeoxychorismate in quantities that enable cell survival in the absence of PabB.