Fig. 1.
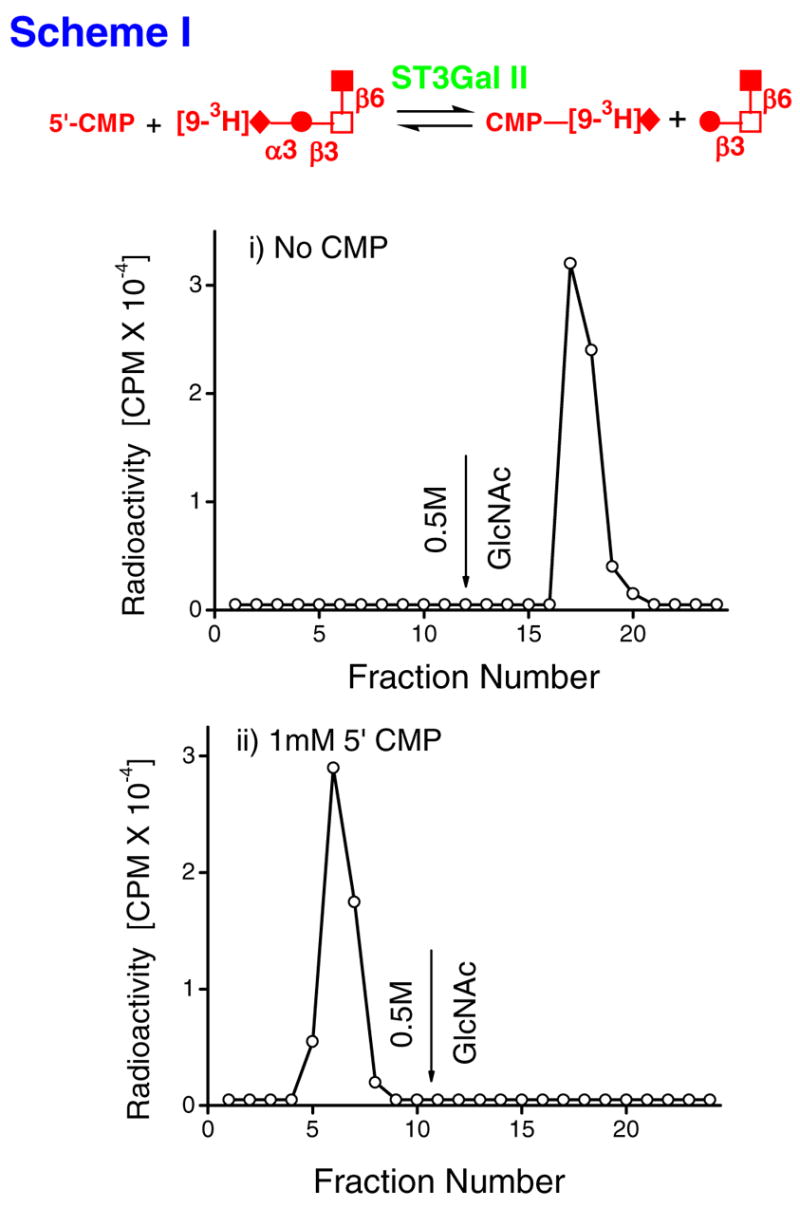
Nucleotide phosphates as acceptors of radioactivity from [9-3H]NeuAcα2,3Galβ,13 (GlcNAcβ1,6)GalNAcα-O-Al (Al=Allyl) [[9-3H][1]] in the presence of ST3Gal-II (scheme-I). Reaction mixtures (RM) with the following composition were incubated at 37ºC for 21h in 100mM NaCacodylate buffer, pH6.0: i) RM containing 150μM (0.4μCi) [[9-3H][1]] (donor) along with 0.8mU ST3Gal-II but no 5′-CMP. ii) RM containing [[9-3H][1]] and 1.0mM 5′-CMP, along with ST3Gal-II. Products formed were fractionated on a WGA-agarose column. Bound products were released using 0.5M GlcNAc at fraction 12. The radioactive product formed in ii) does not bind WGA. Thus, the forward reaction of scheme-I occurs at an appreciable rate. The feasibility of the reverse reaction is well established in literature. Schematic symbols: ◆:sialic acid, ●:Gal, □:GalNAc, ■:GlcNAc
