Fig. 4.
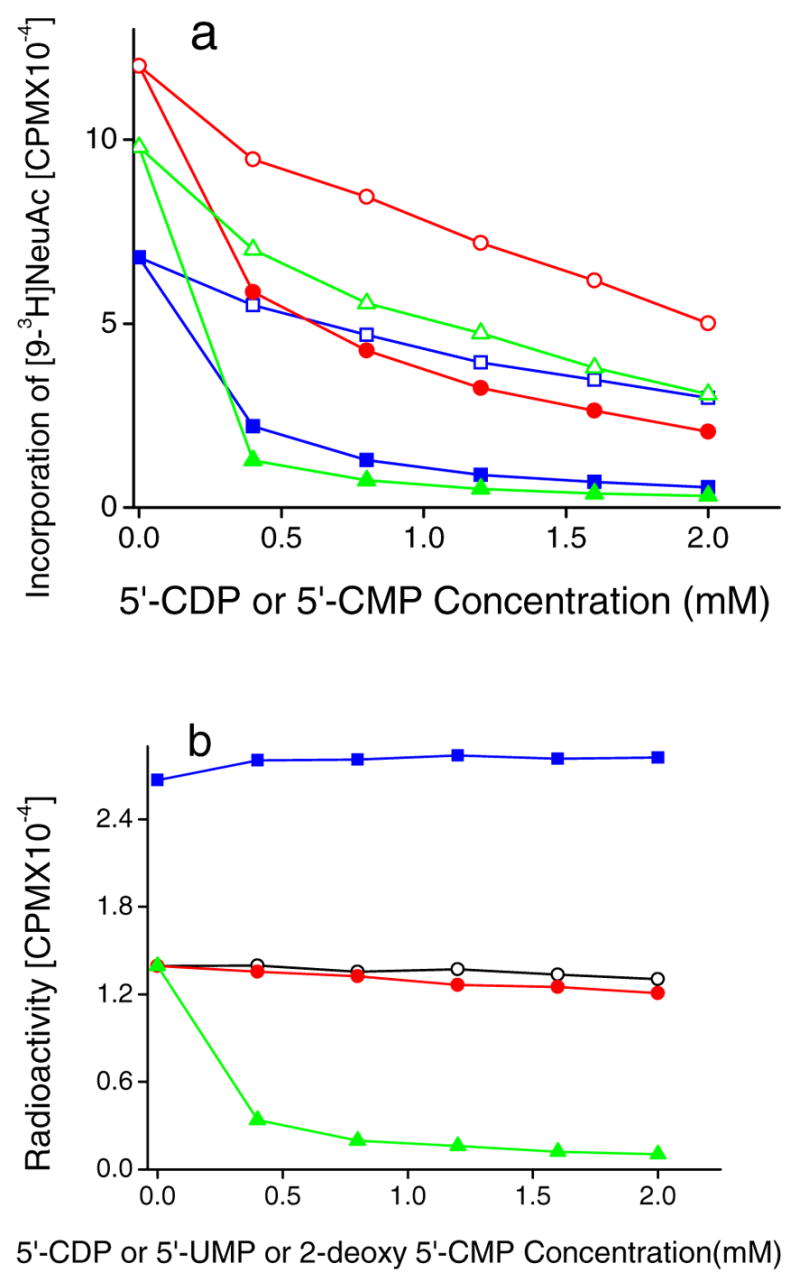
Effect of 5′-nucleotides on direct sialyltransferase activity (panel a) and reverse sialylation (panel b). a. Varying concentrations of 5′-CDP or 5′-CMP were added to 100 mM acceptor (specified below), 0.15mM CMP-[9-3H]NeuAc and either ST3Gal-II (0.2mU), ST3Gal -III (0.5mU) or ST6Gal-I(0.2mU) for 4h at 37ºC. Products were separated using Sep-Pak C18 method. ST3Gal-III activity was measured using acceptor 4-O-MeGalβ1,4GlcNAcβ-O-Bn in the presence of 5′-CDP (●) and 5′-CMP (○). ST6Gal-I activity was measured using Gal β1,4GlcNAcβ-O-Bn in the presence of 5′-CDP (▲) and 5′-CMP (Δ). ST3Gal-II using D-Fucβ1,3GalNAcα-O-Bn [5] in the presence of 5′-CDP (■) and 5′-CMP (□). b) Reverse sialylation by ST3Gal-II was measured in reaction mixtures containing 0.2 mM [9-3H][3], 7mM 5′-CMP, ST3Gal-II (2mU), D-Fucβ1,3GalNAcα-OBn (3.0mM) and varying doses of either 5′-CDP (▲), 5′-UMP (○) or 2-deoxy 5′-CMP (●) for 4h at 37ºC. Reaction product ([9-3H]NeuAcα2,3DFucβ1,3 GalNAcα-O-Bn) was isolated using Sep-Pak C18 method. In some runs, where [5] was absent, the amount of CMP-NeuAc produced was quantified using the Dowex-1 Formate method in the presence of varying doses of 5′-CDP (■)
