Fig. 5.
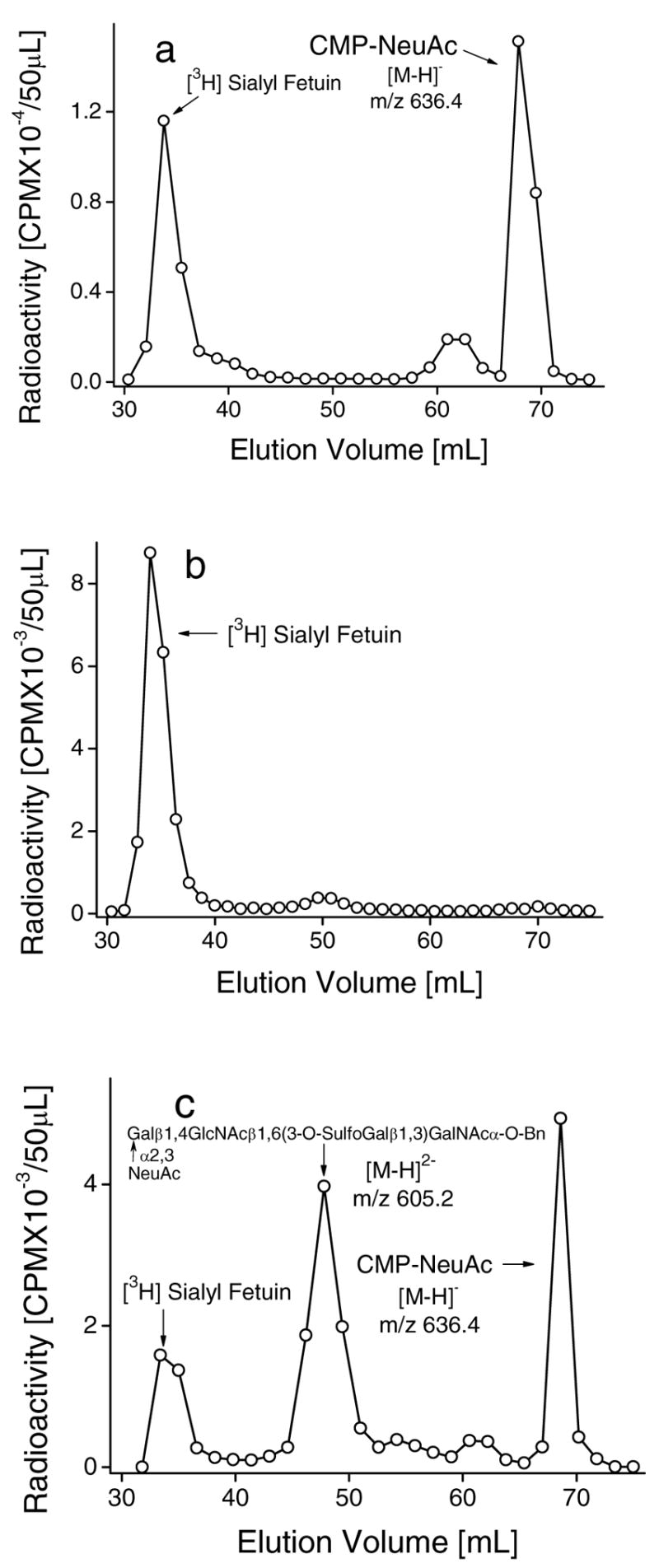
Effect of sialyl or sulfo substituents in O-glycan chain on the reverse sialylation by ST3Gal-II. Incubation mixtures (600μl) contained [9-3H]Sialyl Fetuin (5mg), 200mM NaCacodylate pH 6.0, 20mM 5′-CMP, 50mM ST3Gal-II and the following: a. 6.0mM NeuAcα2,3Galβ1,4GlcNAcβ1,6 (Galβ1,3)GalNAcα-O-Bn, b. 6.0mM 3-O-SulfoGalβ1,4GlcNAcβ1,6(Galβ1,3)GalNAcα-O-Bn or c. 6.0mM Galα1,6GlcNAcβ1,6(3-O-Sulphoβ1,3Gal)GalNAcα-O-Bn along with 50mU ST3Gal-III. Products were fractionated using Biogel P2 column after 20h at 37ºC. Unused [9-3H]Sialyl Fetuin (peak 1) appears prior to [9-3H]Sialyl product from acceptor (peak 2) and CMP-[9-3H]NeuAc (peak 3). Product identities were verified using LC-MS as indicated by molecular weights noted in the panels.
