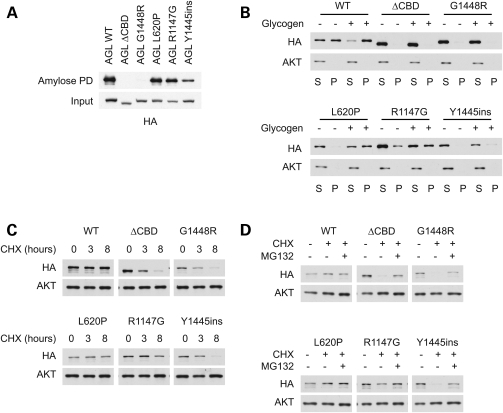
Figure 2.
Effect of amylo-1,6-glucosidase,4-α-glucanotransferase (AGL) mutations on protein stability. (A) Binding of AGL mutants to amylose. Cell lysates expressing HA-tagged AGL proteins were subjected to a pull-down assay using amylose resin. Mutations in the carbohydrate-binding domain (CBD) impair binding to amylose. (B) Ability of AGL proteins to bind to glycogen in a pelleting assay. Cell lysates were fractionated by high-speed ultracentrifugation (100 000g) into a supernatant and a pellet, in the presence or absence of glycogen. Mutations in the CBD domain prevent AGL from pelleting with glycogen. (C) Stability of AGL mutants. Cells expressing HA-tagged AGL proteins were treated with cycloheximide (CHX) to stop protein translation, and the stability of AGL proteins was monitored. (D) Decreased stability of CBD mutants is rescued by proteasomal inhibitors. Cells were treated with CHX for 8 h as in (C) in the presence or absence of MG-132.