Table 1.
Conditions for pyridine formation from dienal 3b
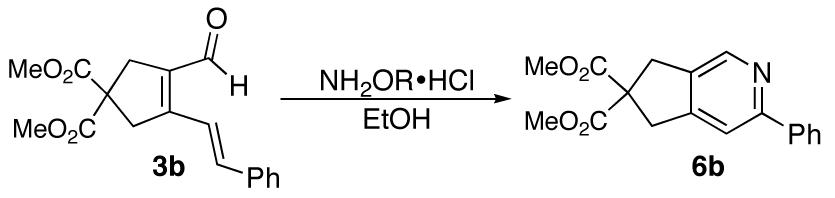 | |||||
|---|---|---|---|---|---|
| entry | R | conditions | time | temp (°C) | yield (6b) |
| 1 | H | NaOAc | 24 h | 90 | 80% |
| 2 | H | NaOAc sealed tube | 4 h | 150 | 82% |
| 3 | H | NaOAc microwave | 1 h | 150 | 98% |
| 4 | Me | NaOAc, microwave | 1 h | 150 | 68% |
| 5(a) | H | pyridine then AcCl | 0.5 h | 0-25 | --(b) |
Pyridine used as solvent
Acetoxy derivative of 4b isolated as only product in 69% yield.
