Table 1.
Selected Optimization Resultsa
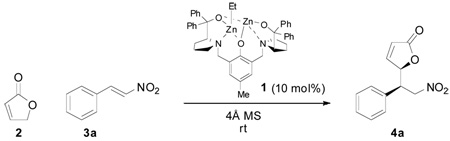 | ||||||
|---|---|---|---|---|---|---|
| entry | solvent | [nitroalkene] | time (h) | yield (%)b | drc | ee (%)d |
| 1 | THF | 0.5 m | 19 | 75 | 8:1 | 91 |
| 2 | toluene | 0.5 m | 8 | 71 | 5:1 | 92 |
| 3 | Et2O | 0.5 m | 26 | 40 | 1:1 | 65 |
| 4 | dioxane | 0.5 m | 22 | 69 | 6:1 | 93 |
| 5 | THF | 0.25 m | 19 | 76 | 10:1 | 91 |
| 6 | THF | 0.125 m | 35 | 71 | 17:1 | 92 |
All reactions were carried out using 1 equiv. of 3a (0.50 mmol), 2 equiv. of 2, 0.10 equiv. of (S,S)-complex 1 and 100 mg 4Å MS.
Refers to the isolated mixture of diastereoisomers.
Determined by 1H NMR of the crude reaction mixture.
Enantiomeric excess of the major diastereoisomer, determined by chiral HPLC.
