Abstract
AIMS
To investigate short-term effects of inhaled salbutamol on haemodynamic changes and cardiovascular autonomic control.
METHODS
A randomized, single-blinded, placebo-controlled study of 0.2 mg of inhaled salbutamol was conducted on 12 healthy nonsmoking volunteers with a mean age of 24 ± 2 years at two different testing sessions. Non-invasively obtained continuous haemodynamic measurements of cardiac output, beat-to-beat arterial blood pressure, and total peripheral resistance were recorded prior to and for a total of 120 min after inhalation of the respective study drug. Continuous cardiovascular autonomic tone was recorded using power spectral analysis of heart rate and blood pressure variability. Spontaneous baroreceptor activity was assessed by the sequence method.
RESULTS
There were no significant changes in any of the baseline parameters between the different testing sessions. Inhalation of salbutamol caused a significant increase in cardiac output from 6.7 ± 1.3 to 7.7 ± 1.4 l min−1 (P < 0.05), and a decrease in total peripheral resistance from 1076 ± 192 to 905 ± 172 dyne s−1 cm−5 (P < 0.05) within 15 min after inhalation. Moreover, salbutamol significantly increased sympathetically mediated low-frequency heart rate variability (P < 0.01), whereas parasympathetically mediated high-frequency heart rate variability decreased (P < 0.01). All changes persisted for approximately 30 min and were fully reversible at 120 min. There were no significant changes in systolic blood pressure variability or spontaneous baroreceptor activity.
CONCLUSIONS
Inhalation of therapeutic doses of salbutamol in healthy subjects resulted in significant haemodynamic changes and a shift of sympathovagal balance towards increased sympathetic tone in the absence of baroreceptor activation.
Keywords: haemodynamics, heart rate variability, inhaled β2-agonist
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Previous reports have demonstrated a link between inhaled β2-agonist use and cardiovascular morbidity and mortality.
The underlying mechanism for this relationship, however, remains controversial.
WHAT THIS STUDY ADDS
Inhalation of a therapeutic dose of salbutamol resulted in significant haemodynamic changes, which were accompanied by a shift in cardiovascular autonomic tone towards increased sympathetic outflow in the absence of baroreceptor activation.
The observed changes in cardiac autonomic function may contribute to an increased cardiac risk associated with inhaled β2-agonist treatment.
Introduction
Selective β2-agonists, such as salbutamol, are in widespread use for patients with asthma or chronic obstructive pulmonary disease (COPD). In addition to their bronchodilating effect, β2-agonists are capable of causing unfavourable effects on the cardiovascular system. A number of previous reports have described a relationship between oral or inhaled β2-agonist use and increased cardiovascular morbidity and mortality [1–7]. Using a case–control study, Au and co-workers [7] have previously shown that patients hospitalized for myocardial infarction or unstable angina were significantly more likely than control subjects to have received prior inhaled β2-agonist treatment. Their findings did not appear to be confounded by tobacco use, COPD history, cardiovascular disease or cardiovascular risk factors, suggesting a direct link between inhaled β2-agonist use and cardiovascular ischaemic events. A number of factors may contribute to the increased cardiovascular risk associated with inhaled β2-agonists: (i) an increase in heart rate due to systemic absorption of the drug, which may result in a shortening of the diastole, thereby increasing myocardial oxygen consumption and reducing the time for coronary artery perfusion; (ii) a decrease in potassium concentrations, which may exert pro-arrythmogenic effects [8]; and (iii) direct effects of salbutamol on β-adrenoceptors of the heart, resulting in increased sympathetic outflow [9]. A recent report by Kallergis and coworkers [10] has furthermore demonstrated that nebulized salbutamol leads to significant electrophysiological effects, such as increased atrioventricular nodal conduction and decreased atrial and ventricular refractoriness.
Despite the large number of clinical studies showing a relationship between inhaled β2-agonist treatment and cardiovascular risk, only few reports have systematically investigated the effects of β2-agonist inhalation on haemodynamic parameters and cardiovascular autonomic control. Quantitative data of cardiovascular autonomic tone can be obtained by spectral analysis of blood pressure and heart rate variability, which have been shown to be independent predictors of cardiovascular morbidity and mortality [11–13]. Thus, the aims of the present study were to assess the acute effects of inhaled salbutamol on cardiovascular autonomic regulation in healthy, nonsmoking volunteers, using continuously obtained haemodynamic measurements, blood pressure and heart rate variability, and baroreceptor activity.
Methods
Test subjects
Twelve healthy nonsmoking volunteers (medical students), seven women and five men, were studied in a prospective, subject-blinded, placebo-controlled study. Subjects were free from any cardiac, respiratory, neurological or hormonal disease and had normal pulmonary function testing (spirometry). None of the subjects was receiving acute or chronic medication. Each participant was informed about the details of the study in a personal interview and all gave their written informed consent. The protocol was approved by the institutional review board.
Experimental setting
Each subject attended two different testing sessions separated by 1 week. An instruction period preceded the first session to familiarize subjects with the use of the study inhaler. Subjects were asked to refrain from heavy exercise for at least 24 h prior to the testing sessions. Alcohol, coffee and tea were prohibited for at least 12 h. The studies were performed between 11.00 and 13.00 h to avoid potential circadian variations in measurements of cardiovascular parameters.
On the study day, subjects were taken to a quiet, dimly lit room. They rested in a supine position on a comfortable bed for ≥20 min to stabilize cardiovascular parameters before starting the measurements. Thereafter, a 15-min recording of baseline parameters was obtained during quiet normal breathing (baseline), which was followed by inhalation of the study drug in a randomized fashion (salbutamol 0.2 mg or placebo with a dummy device). Subjects inhaled the study drug after full expiration and then held their breath for 10 s. A spacer device (ACE-Kit; Smiths Medical ASD, Rockland, MA, USA) was used to maximize drug delivery to the lower airways. After inhalation, subjects were continuously monitored for a total of 120 min. Subjects were questioned about side-effects from the test drug after each testing session.
To assess the individual bronchodilatory effect of the study drug, each participant underwent spirometric measurements of forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio before and after inhalation of either salbutamol or placebo, using a handheld spirometer (Easyone; ndd Medical Technologies, Andover, MA, USA). In order to avoid a potential influence of the breathing manoeuvres on the haemodynamic parameters, spirometric measurements were performed on a separate visit.
Measurements
For monitoring and automatic online calculation of all haemodynamic parameters as well as autonomic cardiovascular tone we used the Task Force Monitor (CNSystems, Graz, Austria). Continuous measurements of systolic (SBP) and diastolic (DBP) beat-to-beat arterial blood pressure were obtained by use of the vascular unloading technique on the finger [14]. Mean arterial blood pressure (mBP) was obtained by integration of the digital pressure waveform. Beat-to-beat blood pressure values were automatically corrected to an offset obtained from oscillometric blood pressure measurements at the contralateral arm (brachial artery). Real-time beat-to-beat stroke volume (SV) was derived using an improved method of transthoracic impedance cardiography [15, 16]. Impedance cardiography utilizes changes in thoracic electrical impedance to estimate changes in blood volume in the aorta and changes in fluid volume in the thorax [15]. By measuring the maximum rate of thoracic electrical impedance during ventricular ejection, dividing it by the base impedance, multiplying by the left ventricular ejection time and a volume constant of the chest (determined by age, weight, height, and body surface area), the SV of the left ventricle can be calculated [15].
Although impedance cardiography may have limits regarding the accuracy of the absolute values of SV and cardiac output (CO), it is a non-invasive tool being increasingly used for physiological and clinical studies when relative changes of haemodynamic recordings are of primary interest [17]. In fact, recent work suggests that measurements as obtained by impedance cardiography are less variable and more reproducible than by the thermodilution technique [18]. Total peripheral resistance (TPR) was calculated as mBP/CO.
Heart rate (HRV) and systolic blood pressure variability (SBPV) were obtained with an adaptive autoregressive model as proposed by Bianchi et al.[19] using a recursive least squares algorithm [20]. Both HRV and SBPV were displayed as three-dimensional sliding power spectra [16]. The total power and the power of user-defined frequency bands were then computed. The defaults were set to three bands: (i) the very low-frequency band between 0 and 0.05 Hz, which are not reported in this study, (ii) the low-frequency band (LF-HRV, LF-SBPV) between 0.05 and 0.17 Hz, and (iii) the high-frequency band (HF-HRV, HF-SBPV) between 0.17 and 0.40 Hz. The power density of each spectral component was calculated both in absolute values (ms2) and normalized units (nu). The ratio between LF- and HFHRV was computed.
In addition, an automatic evaluation of respiratory rate was derived from the impedance signal by extracting the instantaneous respiratory rate without the need for further instrumentation such as spirometric equipment for the chest, mouthpieces or mouth masks. The high-frequency content of the impedance signal was removed by applying a moving average window before further bandpass filtering the resulting signal. Detection of breathing cycles was performed by applying an upper and lower threshold to the respiratory signal and looking for sequences where these thresholds were crossed in the required order to form a complete breathing cycle.
Spontaneous baroreflex activity was evaluated using the sequence method. The sequence method is based on the computer identification in the time domain of spontaneously occurring sequences of consecutive beats in which progressive increases in SBP of ≥ 1 mmHg beat−1 for at least three consecutive heart beats are followed with a one-beat delay by a progressive lengthening in pulse interval (PI) of ≥4 ms beat−1 (PI+/SBP+ sequences) or, vice versa, progressive decreases in SBP are followed by a progressive shortening in PI (PI−/SBP− sequences) [21]. The slope of the regression line between SBP and PI interval changes was taken as an index of the sensitivity of arterial baroreflex modulation of heart rate, as with the laboratory method based on intravenous injection of vasoactive drugs [22]. Using this technique, we have previously demonstrated a reduction in spontaneous baroreceptor activity in response to cardiac preload reduction manoeuvres [23]. Only episodes with correlation coefficients >0.95 were selected, and from all regressions a mean slope of baroreflex sensitivity was calculated for each steady-state period.
Statistical analysis
Results are given as means ± SD and are expressed as absolute values or changes from baseline. Mean values for haemodynamic variables, measurements of HRV and SBPV, baroreflex sensitivity and respiratory rate were calculated as the average of the corresponding time series: baseline, 5 min (T5), 15 min (T15), 30 min (T30) and 90–120 min after inhalation of the study drug (T90). Changes in cardiovascular parameters were examined by analysis of variance for repeated measures (anova). If trends reached statistical significance, a Tukey–HSD-Post-Test correction was performed to determine differences between the respective ventilatory settings. The null hypothesis was rejected at the 5% level.
Results
We studied 12 subjects with a mean age of 24 ± 2 years and body mass index of 22 ± 2 kg m−2. Spirometric results prior to inhalation of the study drug were within normal limits with a FEV1 of 3.9 ± 0.7 l min−1, FVC 4.7 ± 0.8 l min−1, and a FEV1/FVC ratio of 95%. Inhalation of salbutamol did not result in significant changes of the spirometric parameters in the subjects.
There was no evidence of any cardiac arrhythmias after inhalation of the test drugs. After inhalation of salbutamol, however, four subjects (33%) reported slight palpitations, one subject (8%) complained of a headache, and one (8%) perceived a hot flush. No adverse effects were observed after inhalation of placebo.
Acute effects of inhaled salbutamol on haemodynamic measurements
Table 1 demonstrates results of the haemodynamic measurements in the study population. Baseline haemodynamic measurements were similar between the different testing sessions. Overall, inhalation of salbutamol resulted in a significant increase in heart rate from 62 ± 10 to 75 ± 11 beats min−1 (P < 0.05), an increase in CO from 6.1 ± 1.2 to 7.7 ± 1.4 l min−1 (P < 0.05), and a decrease in TPR from 1066 ± 249 to 905 ± 172 dyne s−1 cm−5 (P < 0.05) at T5 after drug administration (Figures 1 and 2). The observed haemodynamic changes peaked at T15, persisted up to T30 and were fully reversible at T90. There were no significant changes in beat-to-beat blood pressure or stroke volume after inhalation of salbutamol, nor were there any significant changes in any of the haemodynamic parameters after inhalation of placebo.
Table 1.
Haemodynamic parameters prior to (baseline) and after inhalation of the study drugs (T5, T15, T30, T90)
| Salbutamol | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | T5 | T15 | T30 | T90 | T5 | T15 | T30 | T90 | |
| HR, beats min−1 | 62 ± 10 | 75 ± 11* | 75 ± 10* | 73 ± 12* | 67 ± 11 | 63 ± 11 | 61 ± 11 | 60 ± 10 | 61 ± 11 |
| SV, ml | 99 ± 15 | 103 ± 14 | 105 ± 17 | 104 ± 17 | 97 ± 14 | 99 ± 14 | 96 ± 14 | 97 ± 13 | 96 ± 15 |
| SBP, mmHg | 116 ± 6 | 116 ± 8 | 117 ± 10 | 117 ± 9 | 117 ± 8 | 115 ± 8 | 115 ± 6 | 114 ± 5 | 117 ± 7 |
| DBP, mmHg | 75 ± 4 | 73 ± 6 | 73 ± 7 | 74 ± 6 | 76 ± 8 | 75 ± 4 | 74 ± 5 | 74 ± 4 | 77 ± 3 |
| CO, l min−1 | 6.1 ± 1.2 | 7.7 ± 1.4* | 7.8 ± 1.4* | 7.5 ± 1.5*† | 6.5 ± 1.3 | 6.1 ± 1.3 | 5.9 ± 1.2 | 5.8 ± 1.1 | 5.8 ± 1.2 |
| CI, l min−1 m−2 | 3.4 ± 0.8 | 4.3 ± 0.8* | 4.4 ± 0.9* | 4.2 ± 0.9† | 3.6 ± 0.8 | 3.5 ± 0.8 | 3.3 ± 0.8 | 3.2 ± 0.7 | 3.3 ± 0.8 |
| TPR, dyne s−1 cm−5 | 1066 ± 249 | 905 ± 172* | 883 ± 192* | 938 ± 195*† | 1109 ± 269 | 1169 ± 287 | 1207 ± 248 | 1203 ± 254 | 1241 ± 244 |
| TPRI, dyne s−1 m−2 cm−5 | 2100 ± 517 | 1624 ± 356* | 1505 ± 575* | 1682 ± 377† | 1991 ± 514 | 2108 ± 597 | 2175 ± 522 | 2166 ± 518 | 2237 ± 522 |
CI, cardiac index; CO, cardiac output; HR, heart rate; SBP, DBP, systolic and diastolic blood pressure; SV, stroke volume; TPR, total peripheral resistance; TPRI, total peripheral resistance index.
P < 0.05 for salbutamol compared with baseline.
P < 0.05 for salbutamol compared with placebo at isotime.
Figure 1.
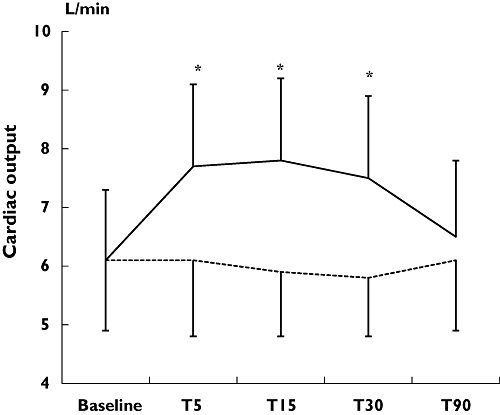
Short-term effects of inhaled salbutamol on cardiac output measurements. T5, T15, T30, T90, indicating 5, 15, 30 or 90 min after inhalation of the study drugs, respectively. *P < 0.05 for salbutamol vs. placebo inhalation. Salbutamol (——); Placebo (······)
Figure 2.
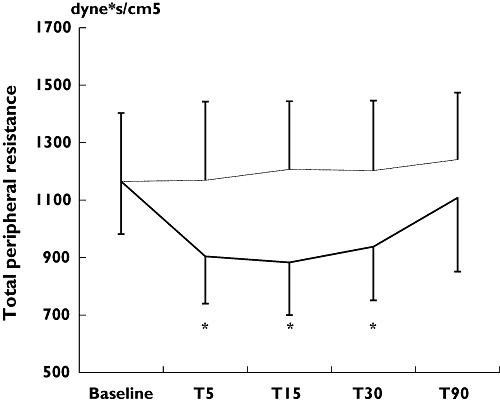
Short-term effects of inhaled salbutamol on total peripheral resistance. T5, T15, T30, T90, indicating 5, 15, 30 or 90 min after inhalation of the study drugs, respectively. *P < 0.05 for salbutamol vs. placebo inhalation. Salbutamol (——); Placebo (······)
Acute effects of inhaled salbutamol on heart rate and blood pressure variability
We observed no significant alterations in respiratory rate following inhalation of either study drug that might have had an influence on changes in HRV. An example of power spectral analysis of HRV is given in Figure 3.
Figure 3.
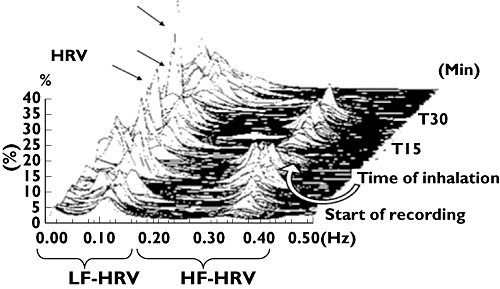
Original tracing from a representative subject during normal breathing and after inhalation of a single therapeutic dose of salbutamol. Drug inhalation resulted in a significant increase in LF-HRV indicating a shift of autonomic tone towards increased sympathetic activity. Peaks are indicated by arrows. LF-HRV and HF-HRV, low-frequency and high-frequency heart rate variability expressed as normalized units. T15, T30, 15 or 30 min after inhalation of the study drug, respectively
There were no significant changes induced by inhalation of either study drug in HRV across the frequency bands when using absolute units (Table 2). Administration of salbutamol, however, significantly decreased parasympathetically mediated HF-HRV expressed in normalized units at T5 (P < 0.05). In contrast, normalized units of sympathetically mediated low-frequency components of HRV were significantly (P < 0.05) increased after inhalation of salbutamol, resulting in an overall increased LF to HF ratio (P < 0.05). The peak of the observed changes in cardiovascular autonomic tone was observed at T5, and similar to the haemodynamic changes was fully reversible at T90.
Table 2.
Heart rate variability and respiratory rate prior to (baseline) and after inhalation of the study drugs (T5, T15, T30, T90)
| Salbutamol | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | T5 | T15 | T30 | T90 | T5 | T15 | T30 | T90 | |
| LFnu-HRV | 43.1 ± 16.6 | 61.0 ± 13.5* | 54.7 ± 13.3 | 51 ± 15 | 46.3 ± 15.1 | 55.3 ± 13.4 | 46 ± 11 | 44.2 ± 11.5 | 43.9 ± 16.0 |
| LF-HRV, ms2 | 2128 ± 2638 | 1248 ± 1208 | 741 ± 553 | 448 ± 388 | 1062 ± 1208 | 2564 ± 2257 | 1740 ± 1764 | 1655 ± 1863 | 1782 ± 2585 |
| HFnu-HRV | 56.9 ± 16.5 | 39.0 ± 13.5* | 45.3 ± 13.3 | 49 ± 15 | 53.7 ± 15.1 | 44.7 ± 13.4 | 54 ± 11 | 55.8 ± 11.5 | 56.1 ± 16.0 |
| HF-HRV, ms2 | 3632 ± 6166 | 1094 ± 1633 | 827 ± 913 | 580 ± 784 | 1753 ± 3255 | 2965 ± 4350 | 2841 ± 4133 | 2727 ± 3575 | 2893 ± 5205 |
| LF/HF ratio | 1.3 ± 1.5 | 2.3 ± 1.6* | 1.7 ± 1.3 | 1.6 ± 1.2 | 1.1 ± 0.7 | 1.4 ± 1.1 | 1.1 ± 0.7 | 0.9 ± 0.5 | 0.9 ± 0.5 |
| Respiratory rate min−1 | 15 ± 3 | 15 ± 4 | 16 ± 3 | 16 ± 3 | 15 ± 4 | 15 ± 3 | 12 ± 3 | 14 ± 3 | 15 ± 3 |
P < 0.05 for salbutamol compared with baseline. LF-HRV and HF-HRV, low-frequency and high-frequency heart rate variability expressed as normalized units (nu) or absolute values (ms2).
Baseline measurements of SBPV were similar between the different testing sessions. There were no significant changes in high- or low-frequency components using absolute or normalized units of SBPV in response to inhalation of the study drugs (Table 3).
Table 3.
Systolic blood pressure variability and spontaneous baroreceptor sensitivity prior to (baseline) and after inhalation of the study drugs (T5, T15, T30, T90)
| Salbutamol | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | T5 | T15 | T30 | T90 | T5 | T15 | T30 | T90 | |
| LFnu-SBPV | 40 ± 12 | 45 ± 13 | 45 ± 13 | 44 ± 10 | 39 ± 9 | 45 ± 14 | 42 ± 14 | 42 ± 14 | 40 ± 13 |
| LF-SBPV, ms2 | 5.9 ± 6.9 | 3.7 ± 2.4 | 2.9 ± 1.8 | 2.5 ± 1.5 | 4.6 ± 4.2 | 4.8 ± 3.3 | 3.8 ± 2.4 | 3.1 ± 1.7 | 5.5 ± 5.2 |
| HFnu-SBPV | 13 ± 14 | 12 ± 11 | 12 ± 10 | 12 ± 9 | 11 ± 5 | 11 ± 6 | 11 ± 6 | 12 ± 6 | 14 ± 6 |
| HF-SBPV, ms2 | 1.6 ± 2.1 | 0.9 ± 0.8 | 0.7 ± 0.7 | 0.6 ± 0.5 | 1.0 ± 0.8 | 1.2 ± 1.0 | 1.1 ± 0.9 | 1.0 ± 0.8 | 1.6 ± 1.0 |
| LF/HF-SBPV | 5.5 ± 4.2 | 6.0 ± 4.0 | 5.9 ± 4.7 | 6.1 ± 5.0 | 4.8 ± 2.9 | 5.2 ± 2.9 | 4.8 ± 2.5 | 4.2 ± 2.3 | 3.3 ± 1.5 |
| PI+/SBP+ | 26 ± 14 | 21 ± 13 | 30 ± 16 | 29 ± 16 | 32 ± 22 | 39 ± 41 | 36 ± 22 | 42 ± 26 | 41 ± 30 |
| PI−/SBP− | 31 ± 14 | 25 ± 11 | 23 ± 13 | 23 ± 13 | 30 ± 25 | 36 ± 22 | 31 ± 20 | 36 ± 24 | 33 ± 22 |
LF-SBPV and HF-SBPV, low-frequency and high-frequency systolic blood pressure variability expressed as normalized units (nu) or absolute values (ms2); PI+/SBP+, PI−/SBP−, sequences of consecutive beats in which progressive increases in SBP of at least 1 mmHg beat−1 for at least three consecutive heart beats are followed by a progressive lengthening in pulse interval (PI) of at least 4 ms beat−1 (PI+/SBP+ sequences) or, vice versa, progressive decreases in SBP are followed by a progressive shortening in PI (PI−/SBP− sequences).
Acute effects of inhaled salbutamol on baroreflex sensitivity
An example of the relationship pulse interval and SBP sequences recorded from a subject prior to and after inhalation of salbutamol is demonstrated in Figure 4. Inhalation of salbutamol did not result in a significant change in spontaneous baroreceptor reflex sensitivity expressed as the mean slope of PI+/SBP+ and PI−/SBP− sequences, as outlined in Table 3.
Figure 4.
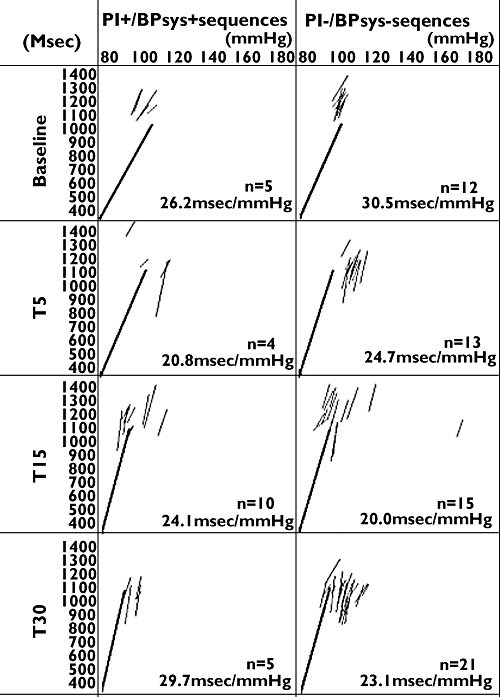
Original record of the relationship between pulse interval (PI) and systolic blood pressure (SBP) in a healthy subject at baseline, 5 (T5), 15 (T15) and 30 (T30) min after inhalation of salbutamol. N, total number of identified spontaneous baroreceptor sequences
Discussion
The present study was designed to investigate short-term effects of inhaled salbutamol on continuously obtained haemodynamic parameters and cardiovascular autonomic function using measurements of heart rate and blood pressure variability.
Salbutamol is an adrenergic agonist whose principle effect is stimulation of β2-receptors with resultant bronchodilation. Compared with oral or parenteral routes of administration, inhalation of salbutamol has reportedly been associated with fewer systemic side-effects for equivalent bronchodilator effect [24–26]. Nevertheless, there is evidence of systemic absorption of the inhaled drug, which may result in clinically significant haemodynamic changes [27]. Most previous studies investigating cardiovascular effects of inhaled bronchodilators, however, have been restricted to measurements of heart rate and/or blood pressure, which may not adequately reflect the full extent of the haemodynamic response that may occur [28–31].
Continuous measurements of heart rate, beat-to-beat arterial blood pressure and cardiac output were used to assess the immediate haemodynamic effects of salbutamol inhalation in healthy subjects in addition to parameters of cardiovascular autonomic tone, including spontaneous baroreceptor activity. Using this approach we observed a significant increase in heart rate and cardiac output in response to inhaled salbutamol, which were accompanied by an increase in sympathetically mediated HRV.
Interpretation of the study results
At least two important pathways may contribute to explain the cardiovascular effects of inhaled salbutamol: (i) direct effects of salbutamol on β2-cardiac adrenoceptors, where they facilitate norepinephrine release [9], and (ii) stimulation of endothelium-dependent nitric oxide-mediated dilation of resistance arterioles resulting in reflex vagal withdrawal and baroreceptor activation [32].
Oscillations in heart rate are being increasingly used as a non-invasive measure of autonomic cardiovascular control [33]. The high-frequency components of HRV result from efferent vagal activity, which has been validated in clinical and experimental observations of autonomic manoeuvres [33, 34]. LF-HRV, in contrast, is increased in conditions known to increase sympathetic outflow such as tilting [35], mental stress [33], infusions with sodium nitroprusside or exercise [35, 36]. Furthermore, there is evidence that spectral analysis of muscle sympathetic nerve activity and HRV share almost identical oscillatory components with a high correlation between both measures in the human subject [37–39]. Blood pressure variability, in contrast, reflects subclinical organ damage independently of absolute blood pressure values rather than acute changes in autonomic cardiovascular control [40, 41].
In the present study we did not observe significant differences in HRV across the frequency bands when absolute units were used; however, when the power of HRV was calculated in normalized units, diminished high-frequency and increased low-frequency HRV was observed. This is of particular importance, since interpretation of changes in the total power of HRV may affect spectral components of low frequency and high frequency expressed in absolute units in the same direction and may prevent appreciation of the fractional distribution of the energy [36]. The observed decrease in normalized units of HF-HRV and increase in normalized units of LF-HRV after salbutamol inhalation may therefore suggest a change in the sympathovagal balance toward increased sympathetic tone [33, 35, 36, 42]. In the absence of significant changes in SBPV and spontaneous baroreceptor activity, our findings are compatible with the hypothesis of a direct effect of salbutamol on cardiac adrenoceptors, rather than baroreceptor activation due to the fall in peripheral resistance.
The effects of inhaled salbutamol on cardiovascular function in previous studies
Using echocardiography to assess haemodynamic changes after inhalation of a single dose of fenoterol in healthy subjects, Chapman et al.[27] found a similar pattern of haemodynamic changes with an increase in heart rate accompanied by a fall in total peripheral resistance. In contrast to our findings, however, the authors reported a wide range in the magnitude of the haemodynamic response among individual subjects, suggesting differential drug delivery to the bronchial tree in that study, which may occur when metered dose inhalers are used without inhaler augmentation devices. Similarly, Waring and coworkers [43] observed an increase in cardiac index and a decrease in systemic vascular resistance after salbutamol inhalation (0.2 mg) in both healthy subjects and pretreated patients with asthma. Extending their findings, we observed that the cardiovascular changes returned to baseline values within 90 min after salbutamol inhalation.
Stimulation of the β2-adrenoceptor further exerts important effects on autonomic cardiovascular control. Administration of salbutamol via the oral route has previously been shown to reduce overall heart rate variability and increase sympathetic components of HRV [44]. Only few reports, however, have investigated the effects of inhaled short acting β2 agonists on HRV. Kaya et al.[30] did not observe significant changes in HRV after inhalation of salbutamol (0.1 mg) in resting healthy probands. Similarly, Dagnone and coworkers [45] did not find any significant changes in heart rate variability after inhalation of higher doses of albuterol (0.4 mg). The lack of monitoring respiratory rate [29, 31], or haemodynamic measurements such as cardiac output [29, 30, 45, 46] and blood pressure [28, 30] in these reports, however, may have prevented observation of important clinical sequels that may be associated with changes in HRV. Furthermore, both above-mentioned studies may have neglected the acute effects of inhaled salbutamol within the first 45 min of application. This is of particular importance, since our findings as well as others [28, 29] suggest that the peak of haemodynamic changes accompanied by alterations in HRV is observed within 15 min of drug delivery. Finally, our study is the first to provide data on the effects of inhaled β2-agonists on both SBPV and spontaneous baroreceptor activity.
Clinical implications
A number of previous studies have reported an association between the use of inhaled β2-agonists and increased cardiovascular morbidity and mortality in patients with both asthma [1, 3–6, 47] and COPD [2]. In a recent meta-analysis of randomized controlled trials of inhaled β2-agonists [48], there was a significantly higher risk for cardiovascular events in the treatment compared with the placebo groups. Our findings may support these observations by providing evidence of altered autonomic cardiovascular tone due to inhaled salbutamol. In fact, the decrease in parasympathetically mediated LF-HRV observed in our report has previously been shown to be predictive of cardiovascular morbidity and mortality [49, 50].
In summary, the present study may suggest that inhalation of a single therapeutic dose of salbutamol results in significant haemodynamic changes in healthy subjects, which are accompanied by a shift in cardiovascular autonomic control towards decreased parasympathetic outflow in the absence of significant changes in spontaneous baroreceptor activity. The observed changes in cardiac autonomic function may contribute to the increased cardiac risk associated with inhaled β2-agonist treatment.
Competing interests
None declared.
REFERENCES
- 1.Crane J, Pearce N, Flatt A, Burgess C, Jackson R, Kwong T, Ball M, Beasley R. Prescribed fenoterol and death from asthma in New Zealand, 1981–83: case–control study. Lancet. 1989;I:917–22. doi: 10.1016/s0140-6736(89)92505-1. [DOI] [PubMed] [Google Scholar]
- 2.Neville E, Corris PA, Vivian J, Nariman S, Gibson GJ. Nebulised salbutamol and angina. BMJ. 1982;285:796–7. doi: 10.1136/bmj.285.6344.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sears MR, Taylor DR, Print CG, Lake DC, Li Q, Flannery EM, Yates DM, Lucas MK, Herbison GB. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–6. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- 4.Sears MR. Short-acting beta-agonist research: a perspective 1997. Can Respir J. 2001;8:349–55. doi: 10.1155/2001/987151. [DOI] [PubMed] [Google Scholar]
- 5.Spitzer WO, Suissa S, Ernst P. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501–6. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 6.Suissa S, Blais L, Ernst P. Patterns of increasing beta-agonist use and the risk of fatal or near fatal asthma. Eur Respir J. 1994;7:1602–9. doi: 10.1183/09031936.94.07091602. [DOI] [PubMed] [Google Scholar]
- 7.Au DH, Lemaitre RN, Curtis JR, Smith NL, Psaty BM. The risk of myocardial infarction associated with inhaled beta-adrenoceptor agonists. Am J Respir Crit Care Med. 2000;161:827–30. doi: 10.1164/ajrccm.161.3.9904006. [DOI] [PubMed] [Google Scholar]
- 8.Pancu D, LaFlamme M, Evans E, Reed J. Levalbuterol is as effective as racemic albuterol in lowering serum potassium. J Emerg Med. 2003;25:13–6. doi: 10.1016/s0736-4679(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 9.Newton GE, Azevedo ER, Parker JD. Inotropic and sympathetic responses to the intracoronary infusion of a beta2-receptor agonist: a human in vivo study. Circulation. 1999;99:2402–7. doi: 10.1161/01.cir.99.18.2402. [DOI] [PubMed] [Google Scholar]
- 10.Kallergis EM, Manios EG, Kanaupakis EM, Schiza SE, Mavrakis HE, Klapsinos NK, Vardas PE. Acute electrophysiologic effects of inhaled salbutamol in humans. Chest. 2005;127:2057–63. doi: 10.1378/chest.127.6.2057. [DOI] [PubMed] [Google Scholar]
- 11.La Rovere MT, Bigger TH, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 12.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–70. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 13.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–6. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 14.Fortin J, Marte W, Grüllenberger R, Hacker A, Habenbacher W, Heller A, Wagner CH, Wach P, Skrabal F. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med. 2006;36:941–57. doi: 10.1016/j.compbiomed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Fortin J, Habenbacher W, Heller A, Hacker A, Grüllenberger R, Innerhofer J, Passath H, Wagner C, Haitchi G, Flotzinger D, Pacher R, Wach P. Non-invasive beat-to beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Comput Biol Med. 2006;36:1185–203. doi: 10.1016/j.compbiomed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Gratze G, Fortin J, Holler A, Grasenick K, Pfurtscheller G, Wach P, Schönegger J, Kotanko P, Skrabal F. A software package for non-invasive, real time beat to beat monitoring of stroke volume, blood pressure, total peripheral resistance and for assessment of autonomic function. Comput Biol Med. 1998;28:121–42. doi: 10.1016/s0010-4825(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 17.Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39:982–8. doi: 10.1161/01.hyp.0000016176.16042.2f. [DOI] [PubMed] [Google Scholar]
- 18.Van de Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance cardiography. The next vital sign technology? Chest. 2003;123:2028–33. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi AM, Mainardi LT, Meloni C, Chierchia S, Cerutti S. Continuous monitoring of the sympatho-vagal balance through spectral analysis. IEEE Eng Med Biol Mag. 1997;16:64–73. doi: 10.1109/51.620497. [DOI] [PubMed] [Google Scholar]
- 20.Schloegl A, Flotzinger D, Pfurtscheller G. Adaptive autoregressive modelling used for single trial EEG classification. Biomed Tech. 1997;42:162–7. doi: 10.1515/bmte.1997.42.6.162. [DOI] [PubMed] [Google Scholar]
- 21.Fritsch JM, Eckberg DL, Graves LD, Wallin BG. Arterial pressure ramps provoke linear increases of heart period in humans. Am J Physiol. 1986;251:R1086–90. doi: 10.1152/ajpregu.1986.251.6.R1086. [DOI] [PubMed] [Google Scholar]
- 22.Watkins LL, Grossman P, Sherwood A. Noninvasive assessment of baroreflex control in borderline hypertension. Comparison with the phenylephrine method. Hypertension. 1996;28:238–43. doi: 10.1161/01.hyp.28.2.238. [DOI] [PubMed] [Google Scholar]
- 23.Valipour A, Schneider F, Kössler W, Saliba S, Burghuber OC. Heart rate variability and spontaneous baroreflex sequences in supine healthy volunteers subjected to nasal positive airway pressure. J Appl Physiol. 2005;99:2137–43. doi: 10.1152/japplphysiol.00003.2005. [DOI] [PubMed] [Google Scholar]
- 24.Larsson S, Svedmyr N. Bronchodilating effect and side effects of ß-2-adrenoceptor stimulants by different modes of administration (tablets, metered aerosol, and combinations thereof); a study with salbutamol in asthmatics. Am Rev Respir Dis. 1977;116:861–9. doi: 10.1164/arrd.1977.116.5.861. [DOI] [PubMed] [Google Scholar]
- 25.Pierce RJ, Payne CR, Williams SJ, Denison DM, Clark TJHA. Comparison of intravenous and inhaled terbutaline in the treatment of asthma. Chest. 1981;79:506–12. doi: 10.1378/chest.79.5.506. [DOI] [PubMed] [Google Scholar]
- 26.Webb J, Rees J, Clark TJ. Comparison of different methods of administration of ß2-sympathomimetics in patients with asthma. Br J Dis Chest. 1982;76:351–7. doi: 10.1016/0007-0971(82)90069-9. [DOI] [PubMed] [Google Scholar]
- 27.Chapman KR, Smith DL, Rebuck AS, Leenen FH. Hemodynamic effects of an inhaled ß2 agonist. Clin Pharmcol Ther. 1984;35:762–7. doi: 10.1038/clpt.1984.108. [DOI] [PubMed] [Google Scholar]
- 28.Eryonucu B, Uzun K, Guler N, Bilge M. Comparison of the acute effects of salbutamol and terbutalin on heart rate variability in adult asthmatic patients. Eur Respir J. 2001;17:863–7. doi: 10.1183/09031936.01.17508630. [DOI] [PubMed] [Google Scholar]
- 29.Jarrti T, Kaila T, Tahvanainen K, Kuusela T, Vanto T, Valikmaki I. The acute effects of inhaled salbutamol on the beat-to-beat variability of heart rate and blood pressure assessed by spectral analysis. Br J Clin Pharmacol. 1997;43:421–8. doi: 10.1046/j.1365-2125.1997.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaya D, Barutcu I, Esen AM, Onrat E, Orman A, Unlu M. Comparison of the effects of ipratropium cromide and salbutamol on autonomic heart rate control. Europace. 2004;6:602–7. doi: 10.1016/j.eupc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Rossinen J, Partanen J, Stenius-Aarniala B, Nieminen MS. Salbutamol inhalation has no effect on myocardial ischemia, arrhytmias and heart-rate variability in patients with coronary artery disease plus asthma or chronic obstructive pulmonary disease. J Intern Med. 1998;243:361–6. doi: 10.1046/j.1365-2796.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- 32.Graves J, Poston L. Beta-adrenoceptor agonist mediated relaxation of rat isolated resistance arteries: a role for the endothelium and nitric oxide. Br J Pharmacol. 1993;108:631–7. doi: 10.1111/j.1476-5381.1993.tb12853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol. 1989;14:1139–48. doi: 10.1016/0735-1097(89)90408-7. [DOI] [PubMed] [Google Scholar]
- 34.Pomeranz M, Macaulay RJB, Caudill MA. Assessment autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol. 1985;248:H151–3. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 35.Malliani A. The pattern of sympathovagal balance explored in the frequency domain. News Physiol Sci. 1999;14:111–7. doi: 10.1152/physiologyonline.1999.14.3.111. [DOI] [PubMed] [Google Scholar]
- 36.Task Force of the European Society of Cardiology, and the North American Society of Pacing and Electrophysiology. Heart Rate Variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 37.Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–31. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- 38.Cogliati C, Magatelli R, Montano N, Narkiewicz K, Somers VK. Detection of low- and high-frequency rhythms in the variability of skin sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;278:H1256–60. doi: 10.1152/ajpheart.2000.278.4.H1256. [DOI] [PubMed] [Google Scholar]
- 39.Pagani M, Montano M, Porta A, Malliani A, Abbound FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–8. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- 40.Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50:325–32. doi: 10.1161/HYPERTENSIONAHA.107.090084. [DOI] [PubMed] [Google Scholar]
- 41.Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, RH F. Syst-Eur investigators. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–7. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat to beat cardiovascular control. Science. 1981;213:220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 43.Waring SW, Leigh RB. Hemodynamic responses to salbutamol and isometric exercise in young adults with mild asthma. Eur J Clin Pharmacol. 2005;61:9–14. doi: 10.1007/s00228-004-0880-7. [DOI] [PubMed] [Google Scholar]
- 44.Silke B, Hanratty CG, Riddell JG. Heart-rate variability effects of beta-adrenoceptor agonists (xamoterol, prenalterol, and salbutamol) assessed nonlinearly with scatterplots and sequence methods. J Cardiovasc Pharmacol. 1999;33:859–67. doi: 10.1097/00005344-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Dagnone AJ, Parlow JL. Effects of inhaled albuterol and ipratropium bromide on autonomic control of the cardiovascular system. Chest. 1997;111:1514–8. doi: 10.1378/chest.111.6.1514. [DOI] [PubMed] [Google Scholar]
- 46.Jartti T. Asthma, asthma medication and autonomic nervous system dysfunction. Clin Physiol. 2001;21:260–9. doi: 10.1046/j.1365-2281.2001.00323.x. [DOI] [PubMed] [Google Scholar]
- 47.Lanes SF, Garcia-Rodriguez LA, Huerta C. Respiratory medications and risk of asthma death. Thorax. 2002;57:683–6. doi: 10.1136/thorax.57.8.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of ß-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–21. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- 49.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–83. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 50.Walters EH, Gibson PG, Lasserson TJ, Walters JA. Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database Syst Rev. 2007;24 doi: 10.1002/14651858.CD001385.pub2. CD001385. [DOI] [PMC free article] [PubMed] [Google Scholar]


