Figure 2.
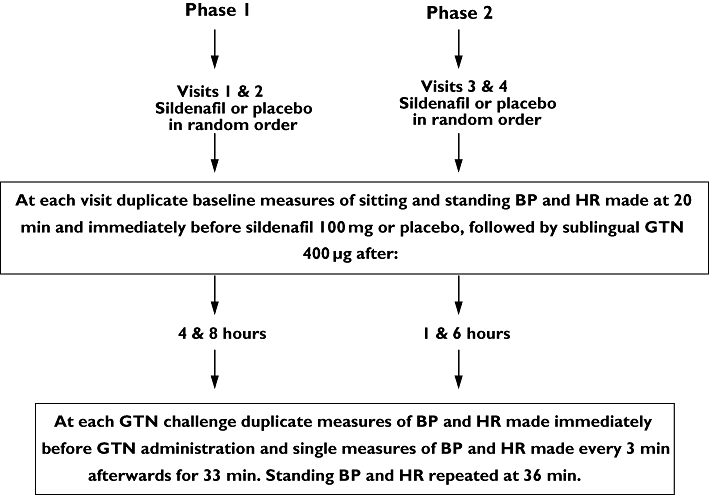
Study 2 was performed in two phases. At each phase subjects attended on two occasions and received, in random order, sildenafil or matched placebo (the alternative therapy was administered after a wash-out of at least 5 days). Sublingual glyceryl trinitrate (GTN) was subsequently administered, after sildenafil or placebo, at the times indicated. Subjects remained in the department throughout the duration of each phase
