Figure 2. Molecular Model of the 83 kDa subunit of CPN.
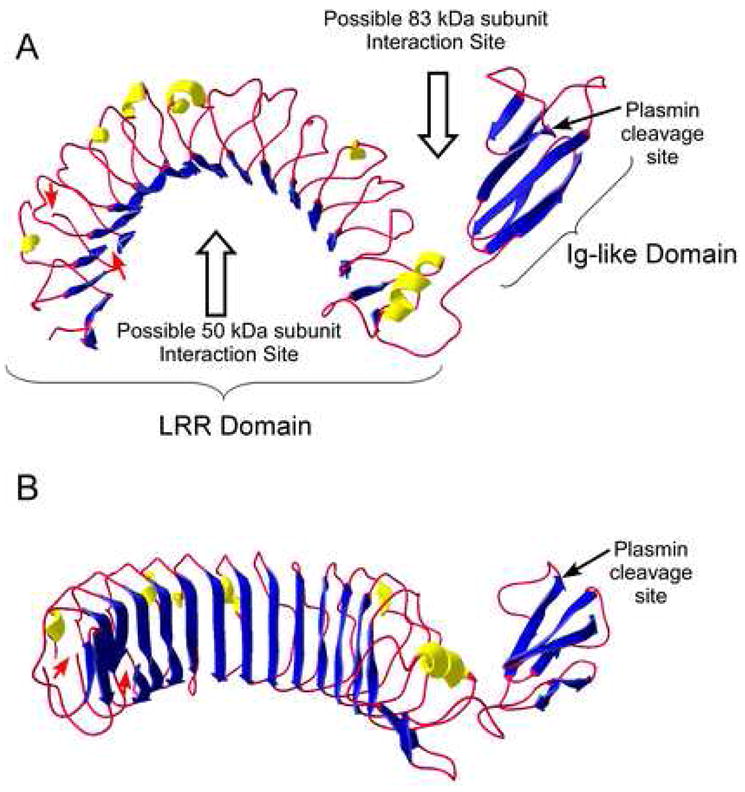
A three-dimensional homology model of the 83 kDa subunit was generated using ESyPred3D [49], based on the structure of Lingo-1 [50]. Helices are colored yellow, β-strands blue and the residual chain as a red rope. Because of additional residues in the 83 kDa subunit (E44– F67 and V83–T106) not found in Lingo-1, the model in the N-terminal region is inaccurate as indicated by gaps in the structure and denoted by the red arrows. The C-terminal 18 residues in the 83 kDa subunit also have no homology with Lingo-1and are not represented in the model. The plasmin cleavage site in the Ig-like domain is indicated by the arrow. A. “Top” view showing the overall curvature of the molecule and the orientation of the C-terminal Ig-like domain. The potential binding sites for the 50 kDa subunit (to form the heterodimer) and another 83 kDa subunit (to form the tetramer) are denoted by the open arrows. B. “Front” view showing the characteristic concave β-sheet surface of the LRR domain.
