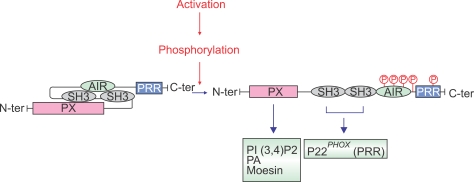
Figure 2.
Phosphorylation of p47phox induces conformational changes and changes domains interactions. In resting state the two p47phox-SH3 domains interact with the C-terminal region AIR to keep the protein in an auto-inhibited state. Upon activation, p47phox is phosphorylated, this phosphorylation induces conformational changes allowing the binding of the cryptic SH3 domains to the proline-rich region (PRR) of p22phox and PX domain to phosphatidylinositol 3,4-biphosphate (PI3,4P), phosphatidic acid (PA) and moesin.