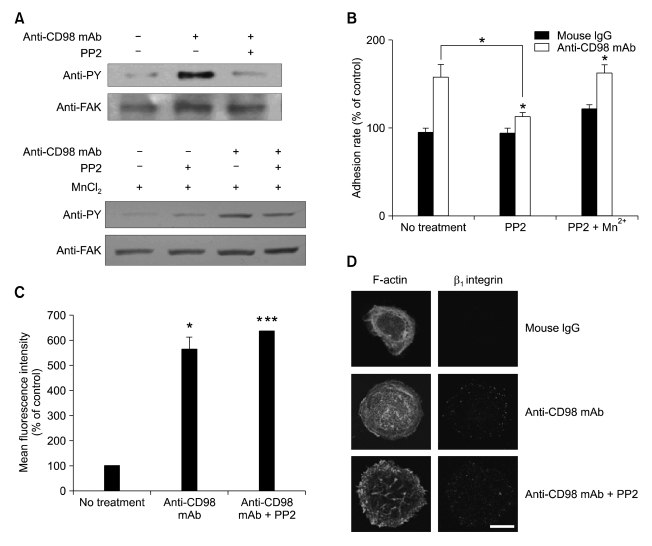
Figure 3.
Inhibition of FAK/Src kinases with PP2 blocks CD98-induced cell adhesion, but not surface expression and clustering of β1 integrins in MCF-7 cells. (A) The effect of PP2 and/or Mn2+ on FAK phosphorylation in MCF-7 cells treated with anti-CD98 mAb was determined by immunoprecipitaion assay. MCF-7 cells were incubated with anti-CD98 mAb and PP2 (0.2 µM) or a DMSO vehicle control in the presence or absence of 0.5 µM Mn2+ for 1 h and anti-FAK rabbit polyclonal antibody (C-20) was used to immunoprecipitate FAK from extracts of MCF-7 cells. Immunoprecipitates were blotted and probed with anti-phosphotyrosine mAb (clone PY99) and anti-FAK mAb. (B) The effect of PP2 treatment on cell adhesion rate was determined as described in Materials and Methods. In addition, whether addition of 0.5 µM Mn2+ interferes with the effect of PP2 on cell adhesion was determined by the same way. Results are expressed as mean ± SE of values relative to the adhesion rate of mouse IgG-treated controls, designated as 100%. Asterisks show a significant difference from control as follows: *P < 0.05. Additional statistical comparisons are indicated by lines. (C) MCF-7 cells were incubated with anti-CD98 mAb with or without PP2 (0.2 µM) and then analyzed by flow cytometry using FITC-conjugated anti-human β1 integrin mAb. Data represent the mean ± SE of values relative to mean values of fluorescence intensity of mouse IgG-treated controls, designated as 100%. Asterisks show a significant difference from control as follows: *P < 0.05, ***P < 0.001 (D) Confocal microscopy was performed as described in Figure 2 legend to investigate the effect of PP2 (0.2 µM) on CD98-induced clustering of β1 integrins. Scale bar, 50 µm. Original magnification, × 400.