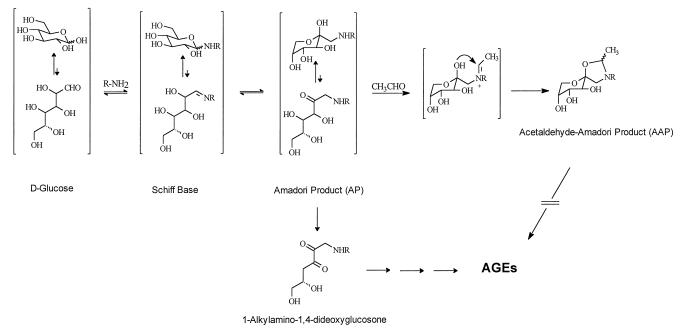
Figure 1.
The early stages of protein glycation showing the formation of the AP from the initial glucose-derived Schiff base adduct. The AP is known to undergo dehydration to a glucosone (diketone), which is the proximate intermediate in the reaction leading to AGE formation and protein crosslinking (7, 8). Acetaldehyde (CH3CHO) reacted with the AP to produce the five-membered AAP (only one stereoisomer is shown). Stabilization of the cyclic form of the AP by acetaldehyde prevents ring opening and the consequent formation of a diketone intermediate leading to AGE crosslinks.