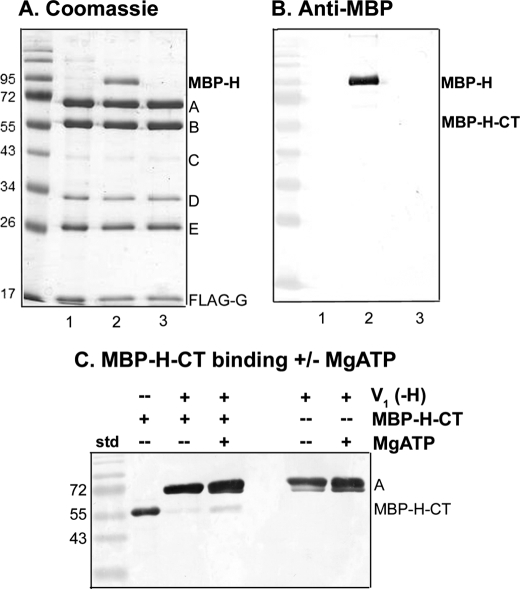
FIGURE 4.
The intact H subunit binds to V1 more tightly than the H-CT fragment. V1(-H) complexes were isolated from vma13Δ yeast cells (lane 1) and incubated with expressed MBP-H (lane 2) or MBP-H-CT (lane 3) followed by reisolation as described in the legend to Fig. 3D. V1(-H) was present at 0.63 μm in each incubation mixture; MBP-H was present at 5.7 μm and MBP-H-CT was present at 19.6 μm. A, Coomassie stained gel of the reisolated V1 complexes is shown. B, an immunoblot with anti-MBP antibody is shown to support identification of MBP-H and the absence of MBP-H-CT after reisolation of complexes as described in Fig. 3D. Note that V1 complexes lacking subunit H were initially isolated by both FLAG affinity chromatography and gel filtration to reduce co-purification of the 95-kDa impurity because it runs near MBP-H. C, V1 complexes were pre-bound to anti-FLAG beads, then incubated with MBP-H-CT (with or without MgATP present) as described under “Experimental Procedures.” After two brief washes to remove unbound MBP-H-CT, the bound material was eluted, and subjected to SDS-PAGE and immunoblotting. The immunoblot was probed with a combination of anti-MBP antibody (1:100,000 dilution) and monoclonal antibody (8B1) against the V1 A subunit (1:10,000 dilution). The MBP-H-CT only sample corresponds to 0.3 μg of purified protein. This blot is representative of four independent experiments.