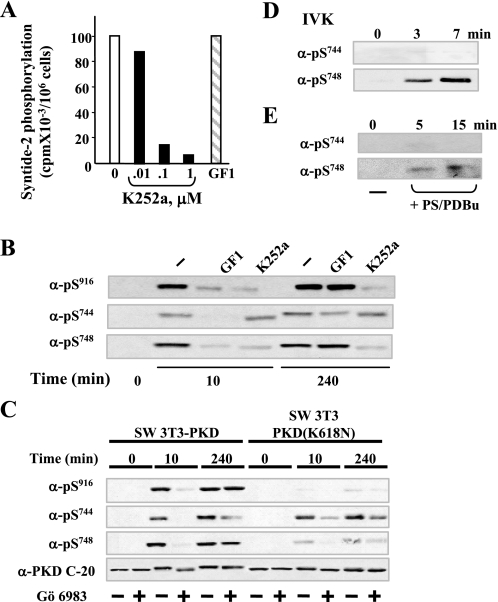
FIGURE 5.
Mechanism of PKD catalytic activation in response to bombesin in Swiss 3T3 cells; differential regulation of Ser744 and Ser748 phosphorylation. A, inhibition of in vitro PKD catalytic activity by K252a. Swiss 3T3 PKD.GFP cells were stimulated with bombesin for 10 min and then lysed in ice-cold buffer. PKD was immunoprecipitated from lysates with an anti-PKD C-20 antibody bound to protein A-agarose and assayed for syntide-2 phosphorylation activity in the presence of various concentrations of K252a (as indicated) or 3.5 μm GF1. B, Swiss 3T3-PKD.GFP were incubated in the absence (–) or in the presence 3.5 μm GF1 or 1 μm K252a as indicated for 1 h before stimulation with 10 nm bombesin for either 10 or 240 min. The cultures were then lysed with 2× SDS-PAGE sample buffer. C, Swiss 3T3-PKD.GFP or Swiss 3T3-PKDK618N.GFP were incubated in the absence (–) or in the presence (+) of 2.5 μm Gö 6983 for 1 h before stimulation of the cells with 10 nm bombesin for the indicated times. The cultures were then lysed with 2× SDS-PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting with the antibodies phospho PKD Ser(P)916, Ser(P)744, Ser(P)748, and PKD-C20 to verify equal loading. The results shown here are representative autoluminograms; similar results were obtained in four independent experiments in panel B and three independent experiments in panel C. D, recombinant purified PKD was incubated with 100 μm ATP in kinase buffer for the times indicated. The reactions were terminated with 2× SDS-PAGE sample buffer. E, PKD eluted from Swiss 3T3 immunocomplexes was incubated with 200 μm ATP in kinase buffer for the times indicated. The reactions were terminated with 2× SDS-PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting with the following antibodies phospho-PKD Ser(P)744 and Ser(P)748. All other details are as described under “Experimental Procedures.” The results shown here are representative autoluminograms; similar results were obtained in two independent experiments.