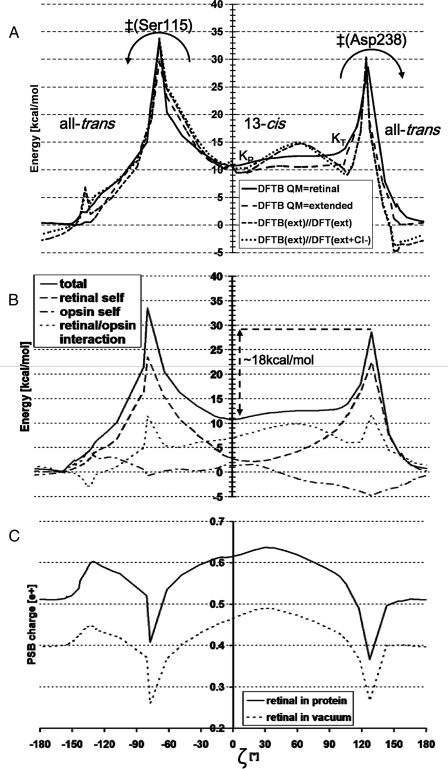
FIGURE 5.
MEP along a 360° rotation of the PSB, trans → 13-cis (via Ser115) → trans (via Asp238). The arrows show the two possible back-isomerization routes. See Fig. 3 for structures along this path and a definition of ζ. The angle ζ is not the driving coordinate, but has been measured in structures taken along the MEP. A, total energy. Optimized energy with DFTB: QM region is retinal only (—) or extended (–––) (see Table 1 for definition). Geometries optimized with DFTB (extended QM region): energy from B3LYP/6–31G* with (···) or without (- - -) the chloride in the QM region. B, energy decomposition (DFTB, QM region is retinal). The total energy (continuous line) is the sum of: the retinal self-energy (dashes), the opsin self-energy (everything excluding retinal, in dash dots), and the interaction between retinal and the opsin (in dots). C, charge (in fraction of one proton charge) on the protonated Schiff base group (see “Experimental Procedures”). Mulliken charges calculated in the presence (continuous line) or absence (dotted line) of the electrostatic field from the protein.