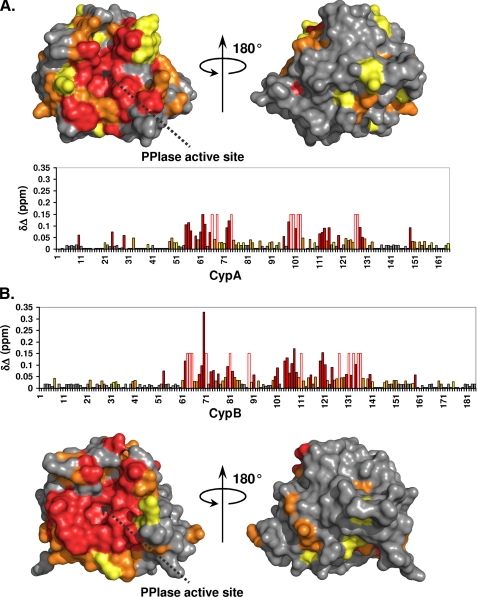
FIGURE 3.
Cyclophilin binding sites for NS5A-D2. The 1H and 15N combined chemical shift perturbations (δΔ) induced on the CypA (A) or CypB (B) spectra following NS5A-D2 addition in a 1:1 molar ratio were plotted along the cyclophilin primary sequences and on their respective three-dimensional molecular surfaces. Residues with combined chemical shift perturbations 0.02 ≤ δΔ ≤ 0.03 ppm are in yellow; 0.03 ≤ δΔ ≤ 0.05 ppm are in orange; and δΔ> 0.05 ppm are in red. For cyclophilin residues for which the proton amide resonances disappear due to important line broadening in the presence of NS5A-D2, a fixed δΔ value of 0.15 ppm was set. These residues are depicted by an open bar circled in red in the diagrams. The PPIase active site of cyclophilins is indicated by a dotted black arrow.