Abstract
Ornithine carbamoyltransferase (OTC) is a key enzyme in the urea cycle to detoxify ammonium produced from amino acid catabolism. OTC deficiency is an X-linked genetic disorder ranging from fatal in newborns to hyperammonemia and anorexia in adults. Through affinity purification of acetylated peptides and mass spectrometry, we identified that OTC is acetylated on lysine residues, including Lys88, which is also mutated in OTC-deficient patients. OTC acetylation was confirmed to occur under physiological conditions. Biochemical characterizations revealed that OTC Lys88 acetylation decreases the affinity for carbamoyl phosphate, one of the two OTC substrates, and the maximum velocity, whereas the Km for ornithine, the other OTC substrate, is not affected. Furthermore, Lys88 acetylation is regulated by both extracellular glucose and amino acid availability, indicating that OTC activity may be regulated by cellular metabolic status. Our results provide an example of the novel mechanism of regulating metabolic enzyme activity through protein acetylation.
Ornithine carbamoyltransferase (OTC)3 is an X-linked mitochondrial enzyme mainly expressed in hepatocytes and enterocytes. OTC is synthesized as a precursor, and the mitochondrial import is accompanied by removal of the N-terminal signal peptide to produce a 36-kDa mature enzyme. As a key urea cycle enzyme, OTC catalyzes the reaction that converts ornithine and carbamoyl phosphate into citrulline. Ornithine is the deamination product of arginine, whereas carbamoyl phosphate is the condensation product of ammonium generated by amino acid deamination and carbon dioxide. Thus, OTC is of crucial importance for cellular ammonium secretion (as a form of urea) and amino acid catabolism (1).
Because there is no alternative way of urea synthesis, blockade in the urea cycle results in devastating health consequences. OTC mutation is a relative common genetic disorder in human. A deficiency of OTC usually results in central nervous system dysfunction, which may cause irreversible brain damage or may be fatal in newborn infants (2). OTC deficiency in adults has milder symptoms but still causes health problems such as hyperammonemia (3, 4) and anorexia (5). Because the gene is on the X chromosome, OTC deficiency is much more prevalent in males. There are >340 OTC mutations identified so far with clinical symptoms (6). No obvious hot spot for OTC mutations has been found. All null mutations are associated with severe neonatal phenotypes, and none survived beyond 5 days of life without treatment. Most disease-associated OTC mutants are missense mutations (7). These observations suggest an indispensable role for OTC activity in human physiology.
Addition of acetyl moiety to the ε-amino group of a lysine residue is a common mechanism of post-translational modification. Protein acetylation regulates various aspects of protein functions, such as regulating protein-protein interaction (8). The role of acetylation in regulating nuclear proteins and transcriptional factors has been well established after more than a decade of intense research. Histone acetylation affects chromatin structure and gene expression (9–11); tumor suppressor protein p53 can be acetylated at multiple sites, and different acetylation modifications have distinct effects on p53 function and physiological impact on the cell (12, 13). The regulatory role of acetylation in enzyme activity was first reported in acetyl-CoA synthetase (14), in which the active site lysine residue in the Salmonella enterica enzyme is acetylated, and therefore, acetylation inhibits acetyl-CoA synthetase activity. Acetyl-CoA synthetase in other species has also been reported to be regulated by acetylation of the active site lysine residue (15, 16), indicating a conserved regulatory mechanism by acetylation for this enzyme.
We performed a proteomic analysis of protein acetylation in human liver. Our studies have identified that lysine 88 in OTC is acetylated. Interestingly, the OTC three-dimensional structure (17–19) shows that Lys88 is not only localized near the carbamoyl phosphate-binding residues (residues 90–93) but that it is also involved in the formation of a complex hydrogen-bonded network that directly participates in substrate binding, indicating a critical role of Lys88 in catalysis. Furthermore, mutation of Lys88 is found in human OTC-deficient patients (20). These two lines of evidence suggest that acetylation on Lys88 may play a key role in the regulation of OTC activity. In this study, we have characterized the OTC acetylation and found that Lys88 acetylation inhibits OTC activity. Furthermore, the Lys88 acetylation is affected by cellular metabolic status, indicating a possible role of acetylation in physiological regulation.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection—HEK293T and Chang liver cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (HyClone), 100 units/ml penicillin, and streptomycin (Invitrogen). Cell transfection was performed using Lipofectamine 2000 (Invitrogen) or calcium phosphate methods.
Polyclonal Antibody Generation—Pan-acetyllysine polyclonal antibodies were generated using chemically modified acetylated chicken ovalbumin as antigen. Polyclonal antibodies against acetylated OTC Lys88 were generated in rabbits using an acetylated peptide (GMIFEK(Ac)RSTRT) as antigen.
Deacetylase Inhibitors Treatment—Deacetylase inhibitor treatments were carried out by adding trichostatin A (TSA; 0.5 μm) and/or nicotinamide (NAM; 5 mm) into culture medium 16 h before harvesting; both concentrations are final concentrations in the culture medium.
Glucose/Amino Acid Treatment—Cells or transfected cells were kept in Dulbecco's modified Eagle's medium for 24 h before treatment. Cells were washed twice with phosphate-buffered saline and continued culture in Dulbecco's modified Eagle's medium (without glucose but with essential amino acids; Sigma, D5030) with the desired supplementation of glucose or amino acids. Amino acid concentrations were the total concentrations of equimolar glutamate and aspartate. Cells were harvested after 12 h of treatment.
Immunoprecipitation—Cells were lysed with lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 50 mm NaF, 1.5 mm Na3VO4, protease inhibitor mixture (Roche), 1 mm dithiothreitol, and 1 mm phenylmethylsulfonyl fluoride). For anti-FLAG immunoprecipitation (IP), 500 μl of cell lysate was incubated with anti-FLAG M2-agarose for 4 h at 4 °C; for OTC antibody IP, lysate was incubated with anti-OTC antibody (Aviva Systems Biology; 1:500) overnight and then protein A/G beads were added, and incubation was continued for another 2 h. Beads were washed three times with lysis buffer, and the FLAG-tagged proteins were eluted by FLAG peptides (Gilson Biochemical).
His-tagged Protein Expression and Purification—His-tagged wild-type OTC, K88Q, and K88R proteins were expressed in Escherichia coli BL21(DE3). After reaching a middle exponential growing stage, 0.2 mm isopropyl 1-thio-β-d-galactopyranoside was added to the bacterial culture to induce protein overexpression. Induction was carried out at 20 °C for 4 h. Expressed proteins were purified with nickel beads (GE Healthcare) as recommended by the manufacturer. Purified proteins were stored at -80 °C in 10% glycerol.
OTC Assay—The OTC enzyme assay was adapted from a published method (21). Briefly, 5 μl of FLAG peptide-eluted ectopic OTC-FLAG solution or 10 μl of OTC antibody-immunoprecipitated beads was added to a solution containing ornithine and triethanolamine to a final volume of 675 μl. OTC reactions were started by adding 75 μl of 150 mm carbamoyl phosphate. Final concentrations in assay of each reagent were 5 mm ornithine, 15 mm carbamoyl phosphate, and 270 mm triethanolamine, pH 7.7. After 30 min of incubation at 37 °C, reactions were stopped by adding 375 μl of phosphoric acid/sulfuric acid (3:1, v/v). Citrulline production was determined by adding 47 μl of 3% 2,3-butanedionemonoxime, boiling in the dark for 15 min, and reading absorbance at 490 nm.
RESULTS
OTC Is Acetylated at Lys88—Most reported acetylation studies are with nuclear proteins, whereas few cytoplasmic protein acetylation studies have been documented. To investigate non-nuclear protein acetylation, human liver tissue was fractionated into nuclear, cytosolic, mitochondrial, and membrane fractions. The subcellular fractions were digested with trypsin, and acetylated peptides were purified by anti-acetyllysine antibody followed by tandem liquid chromatography-tandem mass spectrometry analysis. Among many acetylated peptides identified, the peptide SLGMIFEK*R was identified in both mitochondrial and membrane fractions from liver samples (Fig. 1A). A Sequest Technologies search indicated that this peptide matches human OTC protein and that the acetylated lysine is Lys88. This acetylation peptide was identified in multiple independent experiments. Interestingly, an independent proteomic survey by Kim et al. (22) also reported that the same peptide is acetylated in mouse OTC. Lys88 in OTC is highly conserved in different species from Saccharomyces cerevisiae to human (Fig. 1B), indicating a critical role in OTC function.
FIGURE 1.
OTC is acetylated at Lys88. A, shown is a tandem mass spectrum of the acetylated OTC lysine 88-containing peptide SLGMIFEK*R. B, the acetylated Lys88 in OTC is conserved. The sequences around OTC Lys88 from different species were aligned. Conserved lysine residues corresponding to human OTC Lys88 are boxed. C, transfected OTC is acetylated. HEK293T cells were transfected with pcDNA3 vector, pcDNA3-hOTC-FLAG, and pcDNA3-FLAG-p53 followed by deacetylase inhibitor treatment. Cell lysate was immunoprecipitated with FLAG beads. The precipitated OTC-FLAG was detected by anti-FLAG antibody and anti-acetyllysine antibody (α-AcK) as indicated. NAM and TSA denote treatment with nicotinamide and triochostatin A, respectively. D, endogenous OTC is acetylated. Chang liver cells were treated with deacetylase inhibitors. Endogenous OTC protein was immunoprecipitated with anti-OTC antibody. The acetylation level of endogenous OTC was probed by anti-acetyllysine antibody. E, OTC is acetylated on Lys88. HEK293T cells were co-transfected by pcDNA3-hOTC-FLAG and pcDNA3-hOTCK88Q-FLAG. OTC proteins were precipitated by FLAG beads. OTC Lys88 acetylation levels were detected by an antibody raised against OTC Lys88 peptide.
We are aware of the possibility that lysine acetylation may come from the in vitro manipulation during sample preparation. For example, formic acid used in solubilizing peptides during mass spectrometry assay could be contaminated by trace amounts of acetate anhydrate, and this contamination could cause acetylation of peptides during sample preparation. TSA is a potent inhibitor toward both class I and II deacetylase, whereas NAM can inhibit class III deacetylase (23–25). To confirm that OTC Lys88 is acetylated in vivo, human OTC gene with a FLAG tag at the C terminus was cloned into pcDNA3 and expressed in HEK293T cells, and the transfected cells were treated with deacetylase inhibitors. p53, a well known acetylated protein, was included as a positive control. OTC and p53 proteins were immunoprecipitated from transfected cells followed by Western blotting for lysine acetylation. Interestingly, like p53, TSA and NAM treatment also significantly enhanced OTC acetylation (Fig. 1C). These results confirmed that OTC is acetylated in vivo.
To further examine whether endogenous OTC is acetylated, Chang liver cells were treated with or without TSA plus NAM. Endogenous OTC protein was precipitated with an anti-OTC antibody, and the acetylation level of OTC was probed by antiacetyllysine antibody. We found that the endogenous OTC acetylation level was significantly increased by treatment with TSA and NAM (Fig. 1D). These results indicate that OTC is acetylated under physiological conditions, and the acetylation of OTC is negatively regulated by deacetylases.
Our mass spectrometry data showed that OTC is possibly acetylated on two additional lysine residues, Lys46 and Lys231.A rise in total acetylation detected by anti-acetyllysine antibody is not sufficient to conclude that OTC Lys88 is acetylated. To directly detect OTC Lys88 acetylation, we raised antibody against an acetylated Lys88 OTC peptide. As shown in Fig. 1E, the site-specific antibody recognized the overexpressed wild-type OTC Lys88 acetylation, and the acetylation level of OTC Lys88 increased upon TSA plus NAM treatment. The antibody, however, could not recognize the OTC K88Q mutant, indicating that the antibody is specific for acetylated Lys88 in OTC. The above data demonstrate that Lys88 in OTC is indeed acetylated.
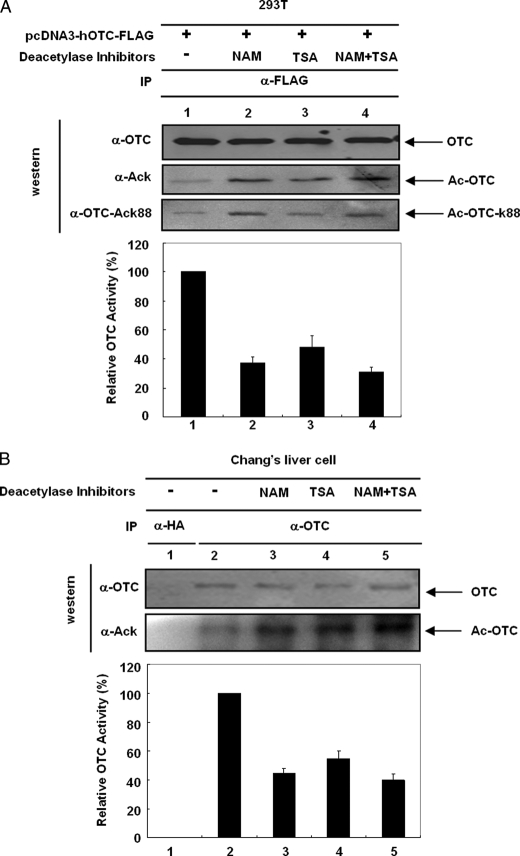
Inhibition of Deacetylase Activity Reduces OTC Activity—Lysine 88 has been implicated to be important for OTC catalysis (26). Therefore, acetylation of Lys88 will likely affect its enzymatic activity. We hypothesize that if OTC Lys88 acetylation regulates its enzyme activity, inhibiting deacetylases could increase the acetylation level of Lys88 and in turn, affect OTC activity. Both endogenous and overexpressed OTC activities were examined to test this hypothesis. We first examined OTC activity regulation by deacetylase inhibitors in transfected HEK293 cells. OTC was transfected into HEK293T cells, and the cells were treated with NAM or TSA. OTC protein was immunopurified, and the activity was measured. We observed that the immunoprecipitated OTC activity was inhibited in cells treated with TSA and NAM (Fig. 2A). An inverse correlation between OTC acetylation and activity strongly indicates that OTC activity is negatively regulated by acetylation. Furthermore, Chang liver cells were cultured in the absence or presence of deacetylase inhibitors, TSA and NAM. Endogenous OTC was immunoprecipitated by OTC antibody-conjugated protein A/G beads, and OTC activity was measured on beads. We found that either TSA or NAM treatment decreased endogenous OTC activity (Fig. 2B). The reduction of OTC activity by TSA and NAM indicates that endogenous OTC activity is inhibited by acetylation.
FIGURE 2.
Inhibition of deacetylase reduces OTC activity. A, deacetylase inhibitors decrease transfected OTC activity. HEK293T cells were transfected with pcDNA3-hOTC-FLAG and treated with deacetylase inhibitors as indicated. OTC proteins were immunoprecipitated with FLAG beads and were eluted by 100 μl of FLAG peptide. OTC assay was carried out, and specific activity was normalized by OTC protein levels determined by Western blotting. Shown are mean ± S.D. of duplicate assays. The overall acetylation level and Lys88 acetylation level were assayed by anti-acetyllysine antibody or anti-acetyllysine-88 antibody. B, NAM and TSA inhibit endogenous OTC activity. Chang liver cells were treated with deacetylase inhibitors. OTC protein was immunoprecipitated with anti-OTC antibody and measure the OTC activity in protein A/G beads. Antihemagglutinin (HA) antibody was used as an IP control. Bars and error bars represent mean ± S.D. of triplicate assays. Specific OTC activities were normalized by the OTC protein level.
Lys88 Acetylation Inhibits OTC Activity—The human disease associated with the K88N mutation causes OTC deficiency, although this mutant OTC has residual activity (27). This is consistent with our finding that inhibition of deacetylases decreases OTC activity. To test the importance of Lys88 acetylation in OTC regulation, we generated the K88R and K88Q mutants. The K88R mutation retains a positive charge and is thus considered as a conserved substitution. K88Q, on the other hand, abolishes the positive charge, and therefore, may mimic the effect of acetylation. Both mutants were expressed in HEK293T cells, and OTC activities were determined from immunoprecipitated protein. The K88R mutant retains substantial enzymatic activity, although it is reduced (Fig. 3A). In contrast, the K88Q mutant is essentially inactive, with <1% of the wild-type OTC activity. These results indicate that a positive charge at position 88 is critical for OTC catalytic activity. Substitution of Lys88 by a glutamine effectively abolishes OTC activity, consistent with our notion that acetylation inhibits OTC activity. We also mutated Lys46 and Lys231, the other two acetylated lysine residues identified in our mass spectrometry data, to glutamines and found that mutation of these two lysine residues had little effect on OTC activity. These results indicate that acetylation of Lys46 and Lys231 is not directly involved in enzyme activity regulation.
FIGURE 3.
Acetylation of Lys88 inhibits OTC activity. A, Lys88 is important for full OTC activity. The wild-type OTC and K88R and K88Q mutants were expressed in HEK293T cells. Proteins were purified by IP, and OTC activity assays were determined. Relative specific OTC activities were normalized by protein level. Wild-type OTC activity was arbitrarily set as 100%. α-Hemagglutinin (HA) antibody was used as an IP control. Bars and error bars represent mean ± S.D. of triplicate assays. B, Lys88 is required for NAM and TSA to repress OTC activity. OTC and the K88R mutant proteins were overexpressed in HEK293T cells followed by deacetylase inhibitor treatment. Proteins were purified by IP, and the total acetylation level, Lys88 acetylation level, and OTC activities were determined, respectively. Relative specific OTC activities were normalized by protein level. α-Hemagglutinin antibody was used as an IP control. Bars and error bars represent mean ± S.D. of triplicate assays.
It is thus of interest to determine whether Lys88 is the primary regulatory acetylation site for OTC activity. We took advantage of the fact that the K88R mutant retains substantial activity. We compared the enzymatic activity of the wild type and the K88R mutant in response to deacetylase inhibitors. As expected, treatment with TSA and NAM decreased OTC enzymatic activity with a concomitant increase in overall OTC acetylation and Lys88 acetylation (Fig. 3B). In contrast, TSA and NAM failed to inhibit the OTC K88R mutant, although overall acetylation was still increased by the deacetylase inhibitors (Fig. 3B). The increase in OTC K88R acetylation is likely due to acetylation of other lysine residues in OTC. Our data strongly indicate that acetylation of Lys88 is responsible for OTC activity inhibition in response to deacetylase inhibitors.
The three-dimensional structure of OTC suggests that a modification at Lys88 may affect OTC substrate binding and thus affect OTC activity. To further investigate the mechanism by which Lys88 acetylation reduces OTC activity, we expressed and purified wild-type OTC and the K88R and K88Q mutants from E. coli. Kinetic studies of the purified OTC show that substitution of Lys88 by either arginine or glutamine does not significantly alter the Km for ornithine, suggesting that Lys88 is not directly involved in ornithine binding (Table 1). In contrast, the K88Q mutant increased the Km of carbamoyl phosphate by 10-fold compared with the wild-type protein, whereas the K88R mutant did not significantly increase the Km. These data suggest that the positive charge residue at position 88 is important for carbamoyl phosphate binding. The above observations indicate that acetylation of Lys88 in OTC may decrease its substrate binding toward carbamoyl phosphate by neutralizing the positive charge of Lys88. Given that the physiological concentration of carbamoyl phosphate is ∼0.1 mm in liver cells (28, 29), the acetylated OTC would have very low activity. It is worth noting that the K88Q mutant not only altered OTC substrate binding but also dramatically decreased the maximum velocity. The OTC K88R mutant also shows a decreased Vmax, although it is much less severe than the K88Q mutant. Our results support a model that OTC activity is negatively regulated by Lys88 acetylation.
TABLE 1.
Kinetic parameters for OTC K88R and K88Q mutants
|
Enzyme
|
Vmax
|
Km
|
||
|---|---|---|---|---|
| Carbamoyl phosphate | Ornithine | |||
| μmol·mg–1·min–1 | mm | |||
| Wild type OTC | 90.9 ± 3.5 | 0.13 ± 0.01 | 0.36 ± 0.13 | |
| OTC K88Q | 0.6 ± 0.1 | 1.24 ± 0.20 | 0.55 ± 0.08 | |
| OTC K88R | 16.7 ± 1.9 | 0.15 ± 0.03 | 0.42 ± 0.09 | |
Lys88 Acetylation Is Influenced by Glucose and Amino Acid Availability—OTC functions in urea cycle and amino acid catabolism, which may be affected by the availability of cellular fuel. We therefore tested the effect of glucose and amino acids on OTC acetylation. We found that lowering the glucose levels led to a decrease in Lys88 acetylation, as determined by the Lys88 acetylation-specific antibody (Fig. 4A). At the same time, OTC activity was increased by lowering glucose levels in cell culture medium (Fig. 4A), consistent with an inhibitory effect of Lys88 acetylation on OTC activity. This result suggests that an increase in Lys88 acetylation may contribute to OTC inhibition by high glucose. We also tested the effect of amino acids (glutamate and aspartate) on OTC acetylation. Surprisingly, higher amino acid concentrations also increased OTC Lys88 acetylation and decreased OTC activity (Fig. 4B). These observations established a direct link between OTC Lys88 acetylation and the availability of extracellular fuels/nutrients, consistent with the notion that OTC acetylation plays a role in cellular metabolic regulation.
FIGURE 4.
Lys88 acetylation is influenced by glucose and amino acid availability. A, glucose increases OTC acetylation and decreases activity. OTC-FLAG proteins were overexpressed in HEK293T cells cultured under different glucose concentrations as indicated. Proteins were purified by IP. OTC activity, OTC acetylation, and Lys88 acetylation were each carried out for purified proteins. Shown are the mean ± S.D. values of triplicate assays for relative OTC activities. Enzyme activity at 0 mm amino acid was arbitrarily set as 100%. All specific activities were normalized by protein levels.B, amino acids increase OTC Lys88 acetylation and decrease activity. OTC-FLAG proteins were overexpressed in HEK293T cells cultured under different amino acid concentrations as indicated. Proteins were purified by IP. OTC activity, OTC acetylation, and Lys88 acetylation were each carried out for purified proteins. Mean ± S.D. values of triplicate assays for relative OTC activities were presented. Enzyme activity at 25 mm glucose was arbitrarily set as 100%. All specific activities were normalized by protein levels.
DISCUSSION
The regulatory role of protein acetylation in gene expression has been well established (30). Interestingly, many metabolic enzymes are also found to be acetylated by proteomic survey in multiple species, ranging from bacteria (31) to human.4 However, the functional significance of acetylation in metabolic enzymes is largely unknown. Besides the mass spectrometry data of identifying acetylation, few metabolic enzymes have been functionally characterized regarding the role of acetylation in physiological regulation. One example is the acetyl-CoA synthetase, which is inhibited by lysine acetylation (15). This report on OTC provides another example that acetylation inhibits the urea cycle enzyme OTC activity by modifying a critical lysine residue in substrate binding and catalysis. Moreover, our data indicate that OTC acetylation is regulated by cellular nutrient signals, as OTC acetylation is enhanced by high glucose or amino acids.
Lysine 88 is the key acetylation site responsible for OTC inactivation. Lys88 locates in a position important for substrate binding/catalysis. Consistently, K88Q has a much weaker affinity toward the substrate carbamoyl phosphate and is almost completely inactive (less than 1% of the wild-type activity). These observations confirm that acetylation may inhibit OTC activity. On the other hand, the K88R mutant had no effect on substrate binding and still retained ∼20% catalytic activity. The moderate reduction in OTC activity of the K88R mutation could be due to the size change in the side chain upon arginine substitution, although the arginine retains the positive charge. On the basis of the mutation analysis and acetylation study, we conclude that acetylation of Lys88 inhibits OTC catalytic activity. It is worth noting that mutation of Lys88 has been found in human OTC deficiency disease, further supporting an important role of Lys88 acetylation in physiological regulation.
Cells utilize glucose as the preferred energy source. When glucose is not abundant, cells shift to alternative energy sources, such as fatty acids and amino acids. Utilization of amino acids as an energy source brings two problems to the cell. First, amino acids are important building blocks for proteins, and biosynthesis of amino acids is energy-expensive; thus, amino acids would not be the preferred energy source. Second, amino acid catabolism produces ammonium, which is toxic to the body. Therefore, cells must adapt to metabolic pathways to get rid of ammonium. The urea cycle is a key pathway for ammonium metabolism. For these reasons, urea cycle enzyme activities need to adapt to different extracellular fuel availability. We found that when glucose concentration is high, OTC Lys88 acetylation is increased, and the OTC activity is turned down (Fig. 4A). Therefore, the urea cycle activity is repressed in the abundance of glucose. This makes physiological sense because cells do not need to catabolize amino acids for energy in the presence of glucose. When glucose concentration is decreased, the increase of OTC activity is possibly due to Lys88 deacetylation. This can be explained by the fact that when cells experience glucose shortage, they need to find an alternative energy source such as amino acids. The detoxification of ammonium generated from amino acid catabolism requires high urea cycle activity. We also observed that inhibition of glycolysis by 2-deoxyglucose inhibited OTC acetylation and concomitantly increased OTC activity (data not shown). Therefore, OTC activity may be regulated by cellular energy status.
OTC Lys88 acetylation is also enhanced by amino acids (Fig. 4B). Consistently, OTC activity is inhibited by high amino acid levels. However, one might expect that high amino acid levels should increase OTC activity due to an elevated demand for the urea cycle. Further studies are needed to clarify the physiological regulation of OTC acetylation in response to amino acids. Important future questions also include how glucose regulates OTC acetylation. We speculate that glucose may regulate OTC acetylation by affecting the Sirt family deacetylase. It is possible that high glucose decreases the cellular concentration of NAD, which is a cofactor for Sirt, and therefore results in a lower Sirt activity and increased OTC acetylation. In summary, our study shows that Lys88 acetylation inhibits OTC enzymatic activity and that this acetylation is regulated by cellular metabolic status and availability of nutrients.
Acknowledgments
We thank Arthur Horwich (Yale University) for providing pOTC plasmid. We thank the members of the Fudan Molecular and Cellular Biology laboratory for discussions throughout this study and the Institutes of Biomedical Sciences for support.
This work was supported by the 985 Program from the Chinese Ministry of Education, the State Key Development Programs of Basic Research of China (Grants 2009CB918401 and 2006CB806700), the National High Technology Research and Development Program of China (Grant 2006AA02A308), the Chinese National Science Foundation (Grants 30600112 and 30871255), and the Shanghai Key Basic Research Project (Grants 06JC14086, 07PJ14011, and 08JC1400900).
Footnotes
The abbreviations used are: OTC, ornithine carbamoyltransferase; NAM, nicotinamide; TSA, trichostatin A; IP, immunoprecipitation; PBS, phosphate-buffered saline.
W. Yu, Y. Lin, J. Yao, W. Huang, Q. Lei, Y. Xiong, S. Zhao, and K.-L. Guan, unpublished data.
References
- 1.Scaglia, F., Brunetti-Pierri, N., Kleppe, S., Marini, J., Carter, S., Garlick, P., Jahoor, F., O'Brien, W., and Lee, B. (2004) J. Nutr. 134 2775S-2782S [DOI] [PubMed] [Google Scholar]
- 2.Hauser, E. R., Finkelstein, J. E., Valle, D., and Brusilow, S. W. (1990) N. Engl. J. Med. 322 1641-1645 [DOI] [PubMed] [Google Scholar]
- 3.Arn, P. H., Hauser, E. R., Thomas, G. H., Herman, G., Hess, D., and Brusilow, S. W. (1990) N. Engl. J. Med. 322 1652-1655 [DOI] [PubMed] [Google Scholar]
- 4.Oechsner, M., Steen, C., Sturenburg, H. J., and Kohlschutter, A. (1998) J. Neurol. Neurosurg. Psychiatry 64 680-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lermansagie, T., and Mimouni, M. (1995) Clin. Pediatr. 34 163-165 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi, S., Brailey, L. L., Morizono, H., Bale, A. E., and Tuchman, M. (2006) Hum. Mutat. 27 626-632 [DOI] [PubMed] [Google Scholar]
- 7.McCullough, B. A., Yudkoff, M., Batshaw, M. L., Wilson, J. R., Raper, S. E., and Tuchman, M. (2000) Am. J. Med. Genet. 93 313-319 [DOI] [PubMed] [Google Scholar]
- 8.Waltzer, L., and Bienz, M. (1998) Nature 395 521-525 [DOI] [PubMed] [Google Scholar]
- 9.Brownell, J. E., and Allis, C. D. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 6364-6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownell, J. E., Zhou, J. X., Ranalli, T., Kobayashi, R., Edmondson, D. G., Roth, S. Y., and Allis, C. D. (1996) Cell 84 843-851 [DOI] [PubMed] [Google Scholar]
- 11.Yang, X. J., Ogryzko, V. V., Nishikawa, J., Howard, B. H., and Nakatani, Y. (1996) Nature 382 319-324 [DOI] [PubMed] [Google Scholar]
- 12.Gu, W., and Roeder, R. G. (1997) Cell 90 595-606 [DOI] [PubMed] [Google Scholar]
- 13.Tang, Y., Zhao, W. H., Chen, Y., Zhao, Y. M., and Gu, W. (2008) Cell 133 612-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starai, V. J., Celic, I., Cole, R. N., Boeke, J. D., and Escalante-Semerena, J. C. (2002) Science 298 2390-2392 [DOI] [PubMed] [Google Scholar]
- 15.Schwer, B., Bunkenborg, J., Verdin, R. O., Andersen, J. S., and Verdin, E. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 10224-10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallows, W. C., Lee, S., and Denu, J. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 10230-10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi, D. S., Morizono, H., Ha, Y., Aoyagi, I., Tuchman, M., and Allewell, N. M. (1998) J. Biol. Chem. 273 34247-34254 [DOI] [PubMed] [Google Scholar]
- 18.Shi, D. S., Morizono, H., Yu, X. L., Tong, L., Allewell, N. M., and Tuchman, M. (2001) Biochem. J. 354 501-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha, Y., McCann, M. T., Tuchman, M., and Allewell, N. M. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 9550-9555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reish, O., Plante, R. J., and Tuchman, M. (1993) Biochem. Med. Metab. Biol. 50 169-175 [DOI] [PubMed] [Google Scholar]
- 21.Ye, X. H., Robinson, M. B., Batshaw, M. L., Furth, E. E., Smith, I., and Wilson, J. M. (1996) J. Biol. Chem. 271 3639-3646 [DOI] [PubMed] [Google Scholar]
- 22.Kim, S. C., Sprung, R., Chen, Y., Xu, Y. D., Ball, H., Pei, J. M., Cheng, T. L., Kho, Y., Xiao, H., Xiao, L., Grishin, N. V., White, M., Yang, X. J., and Zhao, Y. M. (2006) Mol. Cell 23 607-618 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida, M., Kijima, M., Akita, M., and Beppu, T. (1990) J. Biol. Chem. 265 17174-17179 [PubMed] [Google Scholar]
- 24.Bouras, T., Fu, M. F., Sauve, A. A., Wang, F., Quong, A. A., Perkins, N. D., Hay, R. T., Gu, W., and Pestell, R. G. (2005) J. Biol. Chem. 280 10264-10276 [DOI] [PubMed] [Google Scholar]
- 25.Motta, M. C., Divecha, N., Lemieux, M., Kamel, C., Chen, D., Gu, W., Bultsma, Y., McBurney, M., and Guarente, L. (2004) Cell 116 551-563 [DOI] [PubMed] [Google Scholar]
- 26.Valentini, G., De Gregorio, A., Di Salvo, C., Grimm, R., Bellocco, E., Cuzzocrea, G., and Iadarola, P. (1996) Eur. J. Biochem. 239 397-402 [DOI] [PubMed] [Google Scholar]
- 27.Arranz, J. A., Riudor, E., Marco-Marin, C., and Rubio, V. (2007) J. Inherit. Metab. Dis. 30 217-226 [DOI] [PubMed] [Google Scholar]
- 28.Hayase, K., Yonekawa, G., and Yoshida, A. (1992) J. Nutr. 122 1143-1148 [DOI] [PubMed] [Google Scholar]
- 29.Pausch, J., Rasenack, J., Haussinger, D., and Gerok, W. (1985) Eur. J. Biochem. 150 189-194 [DOI] [PubMed] [Google Scholar]
- 30.Allfrey, V. G., Faulkner, R., and Mirsky, A. E. (1964) Proc. Natl. Acad. Sci. U. S. A. 51 786-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, J., Sprung, R., Pei, J., Tan, X., Kim, S., Zhu, H., Liu, C. F., Grishin, N. V., and Zhao, Y. (2009) Mol. Cell. Proteomics 8 215-225 [DOI] [PMC free article] [PubMed] [Google Scholar]