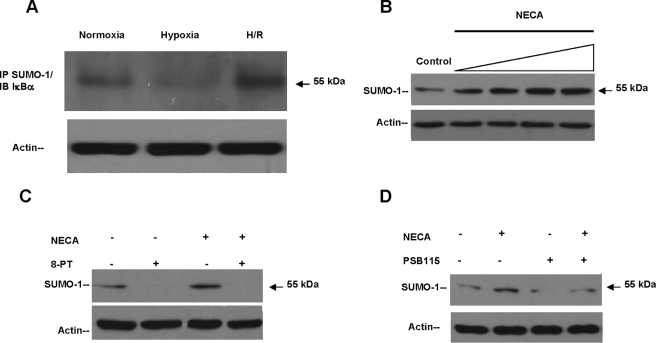
FIGURE 2.
Adenosine signaling promotes SUMOylation of IκBα. A, HeLa cells were exposed to experimental conditions and lysates immunoprecipitated with anti-SUMO-1 antibody and Western blotted against IκBα. A single band of 55 kDa was observed, corresponding to SUMO-1/IκBα isoform. A significant (80%, p < 0.001) increase SUMOylation of IκBα was observed during H/R. Equal loading of samples is documented by actin levels. B, log dose response of NECA (10 nm to 10 μm: 30 min) in HeLa cells with an EC50 estimated to be 100 nm. C, incubation with 8-phenyltheophylline (100 nm; 30 min) resulted in complete inhibition of base-line and NECA-induced protein levels of SUMO-1/IκBα (55 kDa) studied by Western blotting. D, incubation with the Ado A2B specific receptor inhibitor PSB1115 showed ∼89% inhibition of base-line levels of SUMO-1/IκBα and 70% inhibition of the NECA-induced isoform. IP, immunoprecipitation; IB, immunoblot.