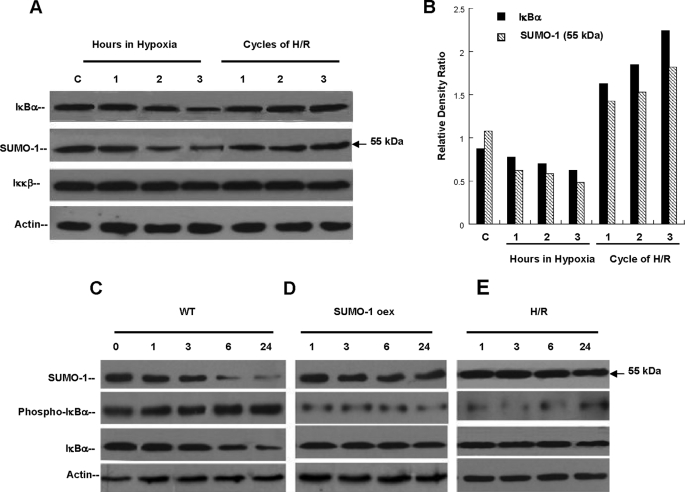
FIGURE 3.
Hypoxia and Reoxygenation prevents IκBα ubiquitination via SUMO-1 modification. A, HeLa cells exposed to 3 h of uninterrupted hypoxia (1% O2) or cycles of H/R. Hypoxia degraded SUMO-1/IκBα (p < 0.01) and unmodified IκBα (p < 0.05) in a similar pattern. No change in protein levels of Iκκβ was observed. In contrast, cycles of H/R totaling 3 h stabilized protein concentrations of SUMO-1/IκBα and prevented the degradation of IκBα with no change in Iκκβ. B, relative density ratios of unmodified and sumoylated IκBα were calculated during hypoxia and H/R. These ratios demonstrate the general degradation effect on both isoforms during continuous hypoxia and demonstrate the cycle-dependent increase provided by increasing cycles of H/R. C, wild type HeLa cells (WT) exposed to 24 h of hypoxia demonstrated significant degradation of SUMO-1/IκBα and unmodified IκBα with early (1 h) phosphorylation of IκBα. D, cells overexpressing the full-length SUMO-1 cDNA exposed to 24 h of hypoxia (1% O2) did not demonstrate evidence of SUMO-1/IκBα or unmodified IκBα degradation despite hypoxia. Relative levels of IκBα phosphorylation were significantly lower than in WT cells exposed to same conditions. E, cells treated with protocols of H/R followed by 24 h of hypoxia. Similarly to SUMO-1-overexpressing cells, H/R prevented degradation of SUMO-1/IκBα or unmodified IκBα and phosphorylation of IκBα.