FIGURE 1.
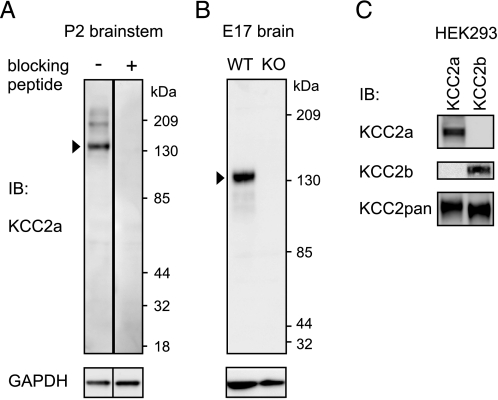
Specific antibodies against the KCC2a and KCC2b isoforms. A, whole-brain lysates obtained from P2 mice were analyzed by standard SDS-PAGE and immunoblotting (IB) with affinity-purified KCC2a antiserum (see “Experimental Procedures”). The KCC2a antiserum (diluted 1: 2000) detects a major product of ∼140 kDa (arrowhead, corresponding to KCC2a monomer), and occasionally (cf. B), a minor band of ∼200 kDa. A similar, higher molecular mass band observed with other KCC2 antibodies was suggested to represent the interaction of KCC2 with other proteins (28). These bands are not observed when the antiserum is preadsorbed with 1 μg/ml immunizing peptide. As a control, the membrane was reblotted with an antibody against GAPDH. B, whole-brain lysates of embryonic day 17.5 (E17) wild-type (WT) mice and their KCC2 null mutant (KO) littermates were analyzed as above. The KCC2a antiserum detected the expected product of ∼140 kDa from the WT but not from the KO lysate. C, lysates of HEK293 cells, transiently transfected with KCC2a or KCC2b expression plasmids, were analyzed as above with KCC2a, KCC2b, or KCC2pan antibodies (see “Experimental Procedures”). The KCC2a antiserum detected a product of ∼140 kDa from lysates of HEK293 cells transfected with the KCC2a plasmid but not with the KCC2b expression vector. Similarly, the KCC2b antiserum stained a product of ∼140 kDa from lysates of KCC2b-expressing but not KCC2a-expressing cells. The KCC2b antibody was raised against a 15-amino-acid peptide corresponding to the N terminus of the KCC2b isoform (11). Because the last five amino acids in the peptide are common to both KCC2 isoforms, the specificity of the antibody for the KCC2b isoform is notable.