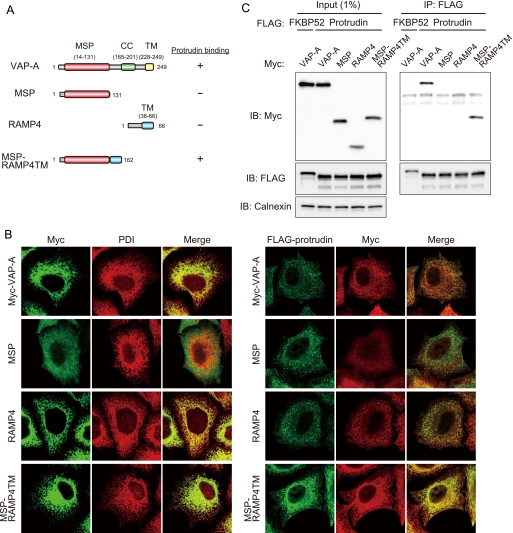
FIGURE 5.
Role of the MSP domain in the interaction of VAP-A with protrudin. A, structure of human VAP-A, the MSP mutant thereof, RAMP4, and the chimeric protein MSP-RAMP4TM as well as a summary of their abilities to bind protrudin as determined in C. B, left panel, HeLa cells expressing 2× Myc-tagged VAP-A, MSP, RAMP4, or MSP-RAMP4TM were fixed and processed for immunofluorescence analysis with anti-Myc (green) and anti-PDI (red). The merged images are also shown. B, right panel, HeLa cells expressing 3× FLAG-protrudin and 2× Myc-tagged VAP-A, MSP, RAMP4, or MSP-RAMP4TM were fixed and processed for immunofluorescence staining with anti-FLAG (green) and anti-Myc (red). The merged images are also shown. C, VAP-A, MSP, RAMP4, or MSP-RAMP4TM fused at their NH2 termini to the 2× Myc tag were expressed in HEK293T cells together with 3× FLAG-tagged protrudin or FKBP52. The cell extracts were subjected to immunoprecipitation (IP) with anti-FLAG, and the resulting precipitates, as well as a portion (1% of the input for immunoprecipitation) of the cell extracts, were subjected to immunoblot analysis with anti-Myc, anti-FLAG, or anti-calnexin. IB, immunoblot.