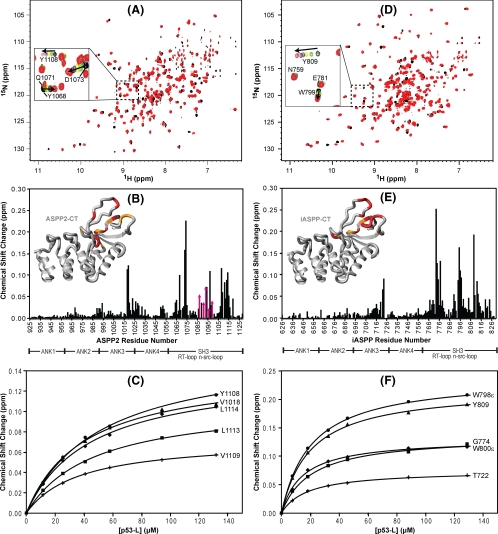
FIGURE 4.
Chemical shift mapping of the interaction between ASPP2-CT and iASPP-CT and the p53 linker peptide. A and D, superposition of the 1H-15N TROSY-HSQC spectra of U-15N,2H-labeled ASPP2-CT (A) and 15N,2H-labeled iASPP-CT (D) in the absence (black) and presence (red) of unlabeled p53-L. A selected region is expanded in each spectrum, and individual resonances along the titration are shown in the inset (increasing amounts of p53-L are shown with the colors black → green → yellow → blue → burgundy → cyan → red). B and E, magnitude of the chemical shift changes versus residue number for ASPP2-CT and iASPP, respectively. Domain boundaries for ASPP2-CT and iASPP-CT are indicated below the residue numbers. Structural mapping of the p53 binding site on ASPP2-CT and iASPP-CT is provided in the insets. The insets show the crystal structures of ASPP2-CT and iASPP-CT (Protein Data Bank codes 1YCS and 2VGE), where residues are colored according to the magnitude of their associated chemical shift changes: red, Δδ > (Δδaverage + 2 × S.D.); orange,(Δδaverage + 2 × S.D.) >Δδ > (Δδaverage + 1 × S.D.). C and F, titration curves for selected ASPP2-CT and iASPP-CT 1HN resonances, respectively.