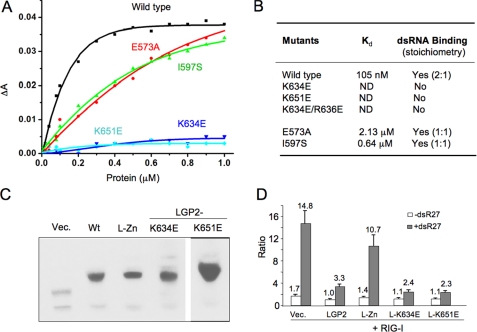
FIGURE 4.
Double-stranded RNA binding is not required for the regulation of RIG-I signaling by LGP2. A, binding studies of LGP2 CTD mutants with a 10-bp dsRNA. Fluorescence anisotropy changes measured by titrating wild type and mutants of LGP2 CTD into Cy3-labeled 10-bp dsRNA. B, summary of dsRNA binding studies of LGP2 CTD mutants by fluorescence anisotropy and gel filtration chromatography. Stoichiometries of the complexes formed between LGP2 CTD and the 8-bp dsRNA are shown in parentheses. C, Western blot analysis of LGP2 and its mutants expressed in the 293T cells. Wt corresponds to wild type LGP2; L-Zn is the double mutant C556A/C559A. D, RNA binding is not required for the inhibition of RIG-I signaling by LGP2. Luciferase assay was performed using HEK 293T cells expressing RIG-I and LGP2 or its mutants at a ratio of 1:2. The data shown are the ratios of the firefly luciferase under the NF-κB promoter versus Renilla luciferase driven from a constitutive herpesvirus thymidine kinase promoter. The white bars correspond to uninduced cells, and the gray bars correspond to cells induced with dsR27 (0.2 μm). LGP2 containing mutations K634E or K651E that abolish dsRNA binding still suppressed RIG-I signaling at levels comparable with that of the wild type protein.