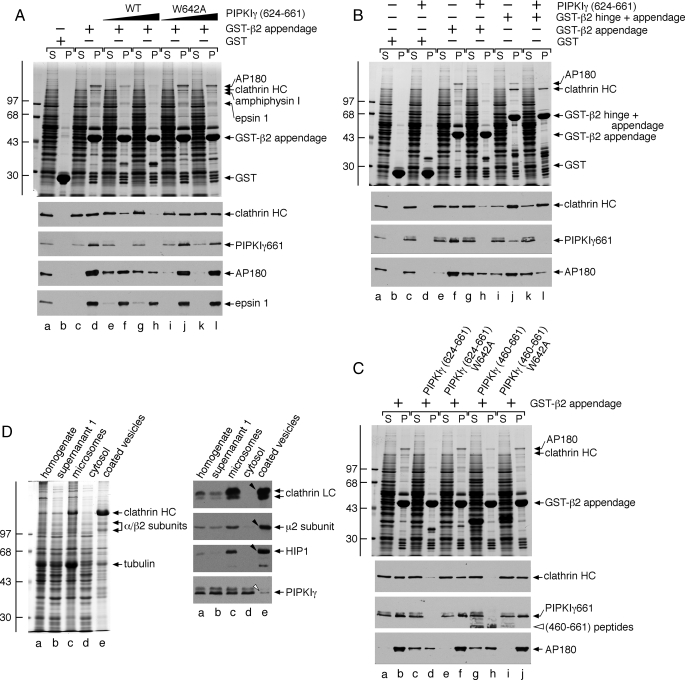
FIGURE 9.
Mutually exclusive engagement of the β2 appendage sandwich by either PIPKIγ661 or clathrin. A, ∼100 μg of GST (lanes a and b) or GST-β2 appendage (lanes c-l) immobilized on glutathione-Sepharose was incubated with rat brain cytosol alone (lanes a-d) or cytosol supplemented with 46 μm wild type (WT; lanes e and f) or W642A (lanes i and j) PIPKIγ-(624-661) peptide or 139 μm wild type (lanes g and h) or W642A (lanes k and l) PIPKIγ-(624-661) peptide. After centrifugation, aliquots of ∼1.5% of each supernatant (S) and ∼10% of each washed pellet (P) were resolved by SDS-PAGE and either stained with Coomassie Blue or transferred to nitrocellulose. Portions of the blots were probed with anti-clathrin heavy chain (HC) mAb TD.1, anti-PIPKIγ mAb clone 12, anti-AP180 mAb clone 34, or affinity-purified anti-epsin 1 antibodies. Notice that although epsin 1 binding to the β2 appendage is affected by the addition of the PIPKIγ peptide, particularly at the highest concentration, the sandwich-binding partners are clearly much more sensitive to the competitor. B, ∼100 μg of GST (lanes a-d), GST-β2 appendage (lanes e-h), or GST-β2 hinge + appendage (lanes i-l) immobilized on glutathione-Sepharose was incubated with rat brain cytosol alone (lanes a, b, e, f, i, and j) or rat brain cytosol supplemented with 113 μm PIPKIγ-(624-661) polypeptide (lanes c, d, g, h, k, and l). After centrifugation, aliquots of ∼1.5% of each supernatant (S) and ∼10% of each washed pellet (P) were resolved by SDS-PAGE and either stained with Coomassie Blue or transferred to nitrocellulose. Portions of the blots were probed with anti-clathrin heavy chain (HC) mAb TD.1, anti-PIPKIγ mAb clone 12, or anti-AP180 mAb clone 34. C, ∼100 μg of GST-β2 appendage immobilized on glutathione-Sepharose was incubated with rat brain cytosol alone (lanes a and b) or cytosol supplemented with 113 μm wild type (lanes c and d) or W642A (lanes e and f) PIPKIγ-(624-661) polypeptide or 113 μm wild type (lanes g and h) or W642A (lanes i and j) PIPKIγ-(460-661) polypeptide. After centrifugation, aliquots of ∼1.5% of each supernatant (S) and ∼10% of each washed pellet (P) were resolved by SDS-PAGE and either stained with Coomassie Blue or transferred to nitrocellulose. Portions of the blots were probed with anti-clathrin heavy chain (HC) mAb TD.1, anti-PIPKIγ mAb clone 12, or anti-AP180 mAb clone 34. D, fractions (20 μg) from a preparation of rat brain clathrin-coated vesicles were resolved by SDS-PAGE and either stained with Coomassie Blue or transferred to nitrocellulose. Portions of the blots were probed with anti-clathrin light chain (LC) mAb Cl57.3, anti-μ2 subunit serum, affinity-purified anti-HIP1 antibodies, or anti-PIPKIγ mAb clone 12. Only the relevant portions are shown. Note the strong enrichment of clathrin, AP-2, and HIP1 (arrowheads) but exclusion of PIPKIγ (open arrowhead) in the coated vesicle fraction.