Abstract
BACKGROUND:
Cystic fibrosis-related diabetes (CFRD) is an increasingly prevalent comorbidity factor for patients with cystic fibrosis (CF). CFRD has been associated with an accelerated decline in clinical parameters and an increased mortality rate.
OBJECTIVES:
To investigate the clinical impact of CFRD on pulmonary function and clinical status using a matched study design to further explore potential causality.
METHODS:
Charts from the adult CF clinic at St Paul’s Hospital (Vancouver, British Columbia) were retrospectively reviewed. Forty CFRD patients with and without fasting hyperglycemia were matched to CF patients with nondiabetic glucose tolerance based on sex, age and forced expiratory volume in 1 s (FEV1).
RESULTS:
Sixteen of 40 CFRD patients (40%) died compared with nine of 40 patient controls (23%) (P=0.13). CFRD patients were more likely to experience declines in FEV1 (P<0.01), especially women (P<0.01). Patients with CFRD were not more likely to be hospitalized (P=0.39). Body mass index did not differ between groups.
CONCLUSIONS:
Patients with CFRD had higher rates of FEV1 deterioration than nondiabetic patients with CF, and showed a trend toward increased mortality. The present study suggests that CFRD has a significant clinical impact and should be carefully considered when evaluating the status of CF patients.
Keywords: Cystic fibrosis, Cystic fibrosis-related diabetes, Forced expiratory volume
Abstract
HISTORIQUE :
Le diabète lié à la fibrose kystique (DLFK) est un facteur de comorbidité de plus en plus prévalent pour les patients atteints de fibrose kystique (FK). Le DLFK s’associe à une détérioration accélérée des paramètres cliniques et à une augmentation du taux de mortalité.
OBJECTIFS :
Explorer les répercussions cliniques du DLFK sur la fonction pulmonaire et l’état clinique au moyen d’une étude appariée pour évaluer la causalité potentielle de manière approfondie.
MÉTHODOLOGIE :
Les dossiers de la clinique de FK pour adultes du St. Paul Hospital (de Vancouver, en Colombie-Britannique) ont fait l’objet d’une étude prospective. Quarante patients atteints de DLFK présentant ou non de l’hyperglycémie à jeun ont été appariés à des patients atteints de DLFK ayant une tolérance au glucose non diabétique d’après le sexe, l’âge et le volume expiratoire maximal par seconde (VEMS).
RÉSULTATS :
Seize des 40 patients atteints de DLFK (40 %) sont décédés par rapport à neuf des 40 sujets témoins (23 %) (P=0,13). Les patients atteints de DLFK étaient plus susceptibles de présenter une diminution du VEMS (P<0,01), notamment les femmes (P<0,01). Les patients atteints de DLFK ne couraient pas plus de risque d’être hospitalisés (P=0,39). L’indice de masse corporelle ne différait pas entre les groupes.
CONCLUSIONS :
Les patients atteints d’un DLFK présentaient des taux de détérioration du VEMS plus élevés que les patients atteints de FK non diabétique et révélaient une tendance vers une mortalité accrue. Selon la présente étude, le DLFK a des répercussions cliniques importantes et devrait être soumis à un examen attentif au moment d’évaluer l’état des patients atteints de FK.
Cystic fibrosis (CF) is the most common life-threatening Caucasian autosomal recessive disorder, with a prevalence of one in 2500 births (1). With improvements in treatment, the median life expectancy of CF patients has increased to 37.0 years (34.8 years in women) (2). CF-related diabetes (CFRD) affects one in four CF patients older than 20 years of age and is the leading comorbidity factor in patients with CF (3,4). Because the median age of onset of CFRD is 20 years, and the life expectancy of the CF population continues to increase, the prevalence of CFRD is expected to grow (3,5). The incidence of CFRD is also higher in women (6–8).
Forced expiratory volume in 1 s (FEV1) is strongly linked to clinical status (9). Pulmonary exacerbations lead to decreased survivorship approximately equal to a 12% drop in FEV1 per admission. In some studies (2,6,8,10), CFRD has been associated with increased mortality and a reduced median life expectancy, while in other studies (11–13) no difference was found. Similarly, some studies (9,14) found that patients with CFRD experienced an accelerated decline in FEV1 two to four years before the diagnosis of diabetes, while another study (15) determined there was no clinical impact. Although the effect of CFRD remains unclear, the severity of glucose intolerance in CF patients correlates with the rate of pulmonary decline and lower pulmonary function variables (3,4,6). However, the above data are primarily cross-sectional and may be consistent with CFRD being only a marker of more severe disease.
Therefore, to more clearly demonstrate the role of CFRD as an independent causative factor for clinical decline, we matched CFRD patients with nondiabetic CF patients to isolate its effect, then assessed their clinical outcomes over the ensuing years.
METHODS
Subjects were identified by a review of 322 patients who attended the adult CF clinic at St Paul’s Hospital (Vancouver, British Columbia) between 1977 and 2005. Patients were classified as CFRD with fasting hyperglycemia (FH), CFRD without FH, impaired glucose tolerance (IGT) or normal glucose tolerance based on existing blood work. An oral glucose tolerance test result of greater than 11.0 mmol/L indicated CFRD, while a result between 7.8 mmol/L and 11.0 mmol/L defined IGT. A fasting blood glucose value of higher than 7.0 mmol/L indicated CFRD with FH, while a fasting blood glucose result of 7.0 mmol/L or lower in patients with CFRD indicated CFRD without FH.
In total, 78 of 322 patients (24.2%) had documented altered glucose tolerance. Of these patients, four were lost to follow-up, four were transferred to another clinic and two had no recorded pulmonary data before death. Of the remaining 68 patients, 14 (20.6%) were eventually diagnosed with IGT and were included in the matching group. Two patients with IGT were used in matching: one was diagnosed with CFRD after the death of her matched patient and the other was diagnosed two years after matching. Five transplant patients and nine patients who had been colonized with Burkholderia cepacia were excluded.
Of patients with normal glucose tolerance, two had no pulmonary data recorded before death, 35 were lost to follow-up and 34 were transferred to a different clinic. Of the remaining patients, 12 (7%) had been colonized with B cepacia and were excluded. The remaining 161 patients together with 14 IGT patients became the matching group.
Forty patients (15 male [38%]), classified as CFRD with FH (34 of 40 patients [85%]) and CFRD without FH (six of 40 patients [15%]), were matched to controls. Cases were matched two years before diagnosis to account for possible effects of the prediabetic phase. Pairs were matched exactly by sex and age, then to the closest FEV1 match. There were no cases in which multiple potential matches had equally close FEV1 values. Only controls born within four years of the CFRD patient in question were considered for matching to eliminate effects from differing therapies over time. For five patients, pulmonary data were not available two years before diagnosis, and matching occurred in the year before diagnosis in three patients and the year of diagnosis in two patients. Patients were identified using a study code identification to minimize possible bias in matching.
The first spirometry measurement in each year was used. Body mass index (BMI) was calculated from the first height and weight measurements in each year.
A Cox proportional hazard model corrected for steroid use was used to determine significance in mortality. FEV1 and BMI were analyzed using a generalized estimate equation corrected for steroid use. A decrease from 0% to 9%, from 10% to 19%, and greater than 20% were used as ordinal categories. Patients who died were considered to be in the highest ordinal group for FEV and forced vital capacity (FVC). Male and female interactions were added to the generalized estimate equation model to analyze sex effects. Admissions were defined as a hospitalization for any cause. The rate of admissions was analyzed using a generalized estimate equation for the prematching period, the matching to diagnosis period and the postdiagnosis period, representing the time points, with none, one, and more than one admission comprising the ordinal categories. Glycosylated hemoglobin A1c (A1C) values were compared in CFRD patients using a repeated measures ANOVA. Analyses were performed using SAS 9.1 (SAS Institute Inc, USA).
Ethics approval was obtained from the University of British Columbia/Providence Health Care Research Ethics Board.
RESULTS
The mean age at diagnosis of CFRD was 24.5 years (men, 25.4 years; women, 23.8 years). Sixteen of 40 patients (40%) with CFRD (five male and 11 female) and nine of 40 controls (23%) (four male and five female) died (hazard ratio=1.918; P=0.1303) with median survival of 10.4 years and 16.14 years after matching, respectively.
Diabetic (23%) therapy, prednisone therapy and BMI at time of matching are presented in Table 1. Eight CFRD patients were on pulses of prednisone, while three were on longer courses. Two patients had allergic bronchopulmonary aspergillosus, one on a pulse and one on a long course of prednisone. Only four cases were taking prednisone between the time of matching and diagnosis of CFRD; the others were prescribed prednisone after CFRD diagnosis. BMI remained unchanged in both groups throughout the study period, and there was no significant difference in BMI between groups (OR=0.889, 95% CI 0.451 to 1.752; P=0.733).
TABLE 1.
Medical treatments and body mass index (BMI) at time of matching
| Treatment | Diabetic patients (n=40) | Control patients (n=40) | P |
|---|---|---|---|
| Insulin, n (%) | 24 (60) | N/A | N/A |
| Metformin and insulin, n (%) | 5 (13) | N/A | N/A |
| Diet, n (%) | 6 (15) | N/A | N/A |
| Sulfonylureas, n (%) | 5 (13) | N/A | N/A |
| Prednisone therapy, n (%) | 11 (28) | 0 (0) | <0.01 |
| BMI, kg/m2 | 20.69 | 21.05 | N/A |
N/A Not applicable
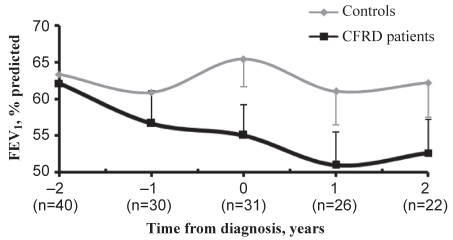
There was no significant difference in FEV1 between groups in the years before matching. However, CFRD patients were more likely to experience decreases in FEV1 between annual measurements (OR=2.947, 95% CI 1.516 to 5.727; P=0.0014). Mean FEV1 for patients with CFRD from the time of matching to four years later were 65.8%, 57.4%, 53.0%, 49.3% and 52.6% compared with 66.3%, 62.2%, 64.0%, 62.3% and 63.7% in controls (Figure 1).
Figure 1).
Mean forced expiratory volume in 1 s (FEV1) over time with standard error. CFRD Cystic fibrosis-related diabetes
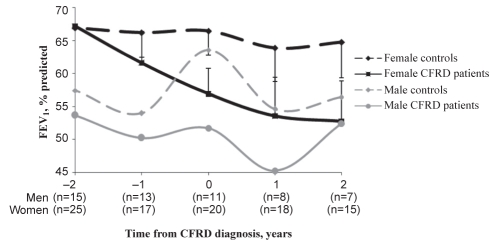
Male CFRD patients were not more likely to experience decreases in FEV1 between annual measurements (OR=1.371, 95% CI 0.468 to 4.014; P=0.565). However, female CFRD patients were more likely to experience decreases in FEV1 between annual measurements (OR=4.409, 95% CI 1.84 to 10.562; P=0.0009). From the time of matching to four years later, female patients had mean FEV1 values of 70.3%, 62.3%, 56.4%, 53.4% and 52.7% compared with 69.4%, 66.8%, 68.3%, 69.8% and 67.5% in controls (Figure 2). Results for FVC over time revealed the same pattern and sex disparities (data not shown).
Figure 2).
Forced expiratory volume in 1 s (FEV1) over time according to sex, with standard error. CFRD Cystic fibrosis-related diabetes
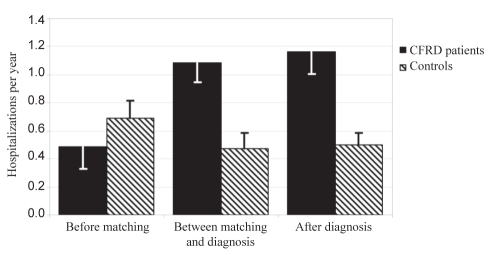
The mean number of hospitalizations per year leading up to matching, from matching to diagnosis and after diagnosis was 0.49, 1.15 and 1.17 for cases and 0.69, 0.47 and 0.50 for controls, respectively. However, after correcting for steroid use, there was no significant difference in the odds of being admitted (OR=1.472, 95% CI 0.612 to 3.54; P=0.3873) (Figure 3). The number of days in the hospital was also not significantly different between groups.
Figure 3).
Mean number of hospitalizations per year with standard error. CFRD Cystic fibrosis-related diabetes
From matching to four years later, the mean A1C value for patients with CFRD was 6.2%, 7.9%, 7.9%, 8.0% and 7.3% (P=0.014). Control values during the same period were 5.6%, 5.2%, 5.6%, 5.5% and 5.4%. Mean A1C was not different between older or more recent calendar years. There was also no significant difference in A1C values between male and female patients.
DISCUSSION
CFRD has been linked to worsened prognosis; therefore, further studying of its effect on patient outcome is clearly clinically relevant. Previous studies have been cross-sectional and thus incapable of determining causality. Many studies (2,4,6) are compilations of registry data, while others used clinic data (11,13,16). Liou et al (10) used registry data to create a survivorship model that they validated with the remaining registry data. All of the above studies were cross-sectional and therefore potentially confounded by the possibility that CFRD only affects sicker patients. In our study, we addressed this limitation by matching individuals by disease severity.
We found that patients with CFRD were more likely to experience a decline in FEV1 and FVC in the years following matching. A1C values remained elevated in the years following diagnosis, suggesting that insufficient treatment or patient non-compliance may have contributed to this finding. Mortality was also nonsignificantly higher in patients with CFRD than the controls in our study. Although the difference did not attain statistical significance in this small sample, the mortality rate has been reported elsewhere to be higher in patients with CFRD (2), while another study (12) reported no difference. However, the latter study was cross-sectional and had a low median survival age, which would obscure the effect of CFRD on mortality. Matching also allowed us to account for patients who died before reaching the median age of onset of CFRD. The importance of matching in assessing CFRD mortality is illustrated by an earlier unmatched assessment of our own clinic’s outcomes, which showed a higher median survival age in CFRD cases.
There is evidence to suggest that pulmonary function is affected even in non-CF patients with diabetes, which may help to explain our findings. Glycosylation of collagen tissue in the lungs reduces elasticity and total capacity (17,18). Dehydration and protein catabolism lead to fatigue and weakness, potentially decreasing pulmonary performance (19,20). Also, microangiopathy decreases gas exchange by reducing capillary blood volume (21,22). Reduction in pulmonary parameters has previously been shown to lead to an increase in all-cause mortality in patients with type 2 diabetes (23). Additionally, patients with diabetes have an impaired ability to fight some infections, have increased rates of colonization and have decreased survivorship (6,24). Certainly these issues would be magnified in the CF cohort and could understandably lead to the serious clinical effects seen.
We found no difference in BMI between groups. However, previous studies (4,6) found that BMI was lower in CFRD patients older than 15 years of age. The difference in our results could be caused by the anabolic effect of insulin therapy; however, A1C values remained elevated suggesting that the effect of insulin therapy was limited and another factor may be responsible. The difference could also be caused by more aggressive nutritional therapy. Alternatively, matching patients may have removed the difference between groups because CFRD patients were likely sicker than controls in those studies. It is also possible that our study size was not large enough to distinguish the difference found in previous studies. We also found that patients with CFRD were not more likely to be hospitalized after correcting for steroid treatment.
The decline in pulmonary parameters and trend toward increased mortality was only significant in female patients. While the lack of effect in males could have been, in part, due to the lower number of male patients, previous studies also found a difference only in female patients for FEV (25) and mortality (16). This finding suggests that female CF patients are more severely affected by CFRD, which could account for a portion of the sex gap in overall CF survival. Some authors have proposed that the effects of gonadal steroids on pulmonary status may be responsible (16).
Our study has several limitations. Because we relied on existing data to classify patients, some CFRD patients may have been missed or misclassified as controls. Two patients later diagnosed with IGT were knowingly used in the control group; therefore, dysglycemia contaminated the control group to at least a small degree. Patients with B cepacia were excluded; the prevalence of which was higher in CFRD patients. Five patients could not be matched two years before diagnosis, thus failing to take into account the prediabetic decline. However, all of the above limitations would have only reduced the differences seen between groups. Patients with CFRD also had a higher rate of corticosteroid use at baseline, suggesting that they may have been in worse clinical condition in spite of similar FEV1 values at matchup. However, FEV1 appeared similar in the years leading to matchup (data not shown), suggesting that both groups were trending downward at a similar rate, and steroid use was included as a correcting factor. Accordingly, we believe that our results were not substantially affected by any of the above limitations.
CONCLUSIONS
We have shown that patients with CFRD have accelerated worsening of pulmonary parameters, and a trend toward increased mortality rates compared with equivalent CF controls with normal glycemic tolerance. Female patients seemed to be more severely affected. The difference in clinical outcome observed suggests that early identification of CFRD should be a priority for any CF care centre.
Acknowledgments
Special thanks to Janet Hopkins, who shared her expertise and facilitated access to the data at the adult CF clinic of St Paul’s Hospital. The authors also thank the Endocrine Research Society and St Paul’s Hospital Foundation for their support.
REFERENCES
- 1.Brennan AL, Geddes DM, Gyi KM, Baker EH. Clinical importance of cystic fibrosis-related diabetes. J Cyst Fibros. 2004;3:209–22. doi: 10.1016/j.jcf.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cystic Fibrosis Foundation . Report of the Canadian Patient Data Registry. Toronto: Canadian Cystic Fibrosis Foundation; 2002. [Google Scholar]
- 3.Lanng S, Thorsteinsson B, Lund-Andersen C, Nerup J, Schiøtz PO, Koch C. Diabetes mellitus in Danish cystic fibrosis patients: Prevalence and late diabetic complications. Acta Paediatr. 1994;83:72–7. doi: 10.1111/j.1651-2227.1994.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 4.Koch C, Rainisio M, Madessani U, et al. Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: Data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol. 2001;32:343–50. doi: 10.1002/ppul.1142. [DOI] [PubMed] [Google Scholar]
- 5.Mackie AD, Thornton SJ, Edenborough FP. Cystic fibrosis-related diabetes. Diabet Med. 2003;20:425–36. doi: 10.1046/j.1464-5491.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 6.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146:681–7. doi: 10.1016/j.jpeds.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 7.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein SM, Wielinski CL, Elliott GR, et al. Diabetes mellitus associated with cystic fibrosis. J Pediatr. 1988;112:373–7. doi: 10.1016/s0022-3476(88)80315-9. [DOI] [PubMed] [Google Scholar]
- 9.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162:891–5. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 10.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reisman J, Corey M, Canny G, Levison H. Diabetes mellitus in patients with cystic fibrosis: Effect on survival. Pediatrics. 1990;86:374–7. [PubMed] [Google Scholar]
- 12.Thornsteinsson B, Lanng S, Nerup J, Koch C. Epidemiology of glucose intolerance in cystic fibrosis. Diabetes. 1991;40:320A. [Google Scholar]
- 13.Rodman HM, Doershuk CF, Roland JM. The interaction of 2 diseases: Diabetes mellitus and cystic fibrosis. Medicine (Baltimore) 1986;65:389–97. doi: 10.1097/00005792-198611000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Rolon MA, Benali K, Munck A, et al. Cystic fibrosis-related diabetes mellitus: Clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr. 2001;90:860–7. [PubMed] [Google Scholar]
- 15.Cucinotta D, Arrigo T, De Luca F, et al. Metabolic and clinical events preceding diabetes mellitus onset in cystic fibrosis. Eur J Endocrinol. 1996;134:731–6. doi: 10.1530/eje.0.1340731. [DOI] [PubMed] [Google Scholar]
- 16.Milla C, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28:2141–4. doi: 10.2337/diacare.28.9.2141. [DOI] [PubMed] [Google Scholar]
- 17.Schnider SL, Kohn RR. Glucosylation of human collagen in aging and diabetes mellitus. J Clin Invest. 1980;66:1179–81. doi: 10.1172/JCI109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange P, Groth S, Kastrup J, et al. Diabetes mellitus, plasma glucose and lung function in a cross-sectional population study. Eur Respir J. 1989;2:14–9. [PubMed] [Google Scholar]
- 19.Ferry M. Strategies for ensuring good hydration in the elderly. Nutr Rev. 2005;63:S22–9. doi: 10.1111/j.1753-4887.2005.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Weekers F, Giulietti AP, Michalaki M, et al. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003;144:5329–38. doi: 10.1210/en.2003-0697. [DOI] [PubMed] [Google Scholar]
- 21.Sandler M. Is the lung a ‘target organ’ in diabetes mellitus? Arch Intern Med. 1990;150:1385–8. [PubMed] [Google Scholar]
- 22.Sinha S, Guleria R, Misra A, Pandey RM, Yadav R, Tiwari S. Pulmonary functions in patients with type 2 diabetes mellitus & correlation with anthropometry & microvascular complications. Indian J Med Res. 2004;119:66–71. [PubMed] [Google Scholar]
- 23.Davis WA, Knuiman M, Kendall P, Grange V, Davis TM. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: The Fremantle Diabetes Study. Diabetes Care. 2004;27:752–7. doi: 10.2337/diacare.27.3.752. [DOI] [PubMed] [Google Scholar]
- 24.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 25.Sims EJ, Green MW, Mehta A. Decreased lung function in female but not male subjects with established cystic fibrosis-related diabetes. Diabetes Care. 2005;28:1581–7. doi: 10.2337/diacare.28.7.1581. [DOI] [PubMed] [Google Scholar]