Abstract
Using an immunocytochemical staining assay involving six different recombinant baculoviruses with each expressing one of the major bovine rotavirus VP7 (G6, G8 and G10) and VP4 (P6[1], P7[5] and P8[11]) serotypes, we analyzed IgG antibody responses to individual proteins in archival serum samples collected from 31 calves monthly from 1 month to 12 months of age during 1974–1975 in Higley, Arizona. Seroresponses to VP7 and VP4, as determined by a 4-fold or greater antibody response, were not always elicited concurrently following infection: in some calves, (i) seroresponses to VP7 were detected earlier than to VP4 or vice versa; and (ii) a subsequent 2nd seroresponse was detected for VP7 or VP4 only. In addition, a second infection was more likely to be caused by different G and/or P types. Analyses of serum samples showed that the most frequent G-P combination was G8P6[1] followed by G8P7[5], G8P8[11] and G6P6[1].
Keywords: rotavirus, bovine rotavirus, serotype, diarrhea, immune response
INTRODUCTION
Group A rotaviruses, members of the genus Rotavirus within the family Reoviridae, are a common cause of acute infectious diarrheal illness in infants and the young of a wide variety of mammalian and avian species [14]. Bovine rotaviruses are important causative agents of neonatal calf diarrhea throughout the world, and rotavirus infection is a significant cause of economic loss in the cattle industry [58]. The rotavirus genome, which consists of 11 segments of double stranded RNA (dsRNA), encodes six structural viral proteins (VP1-4, 6 and 7) and six non-structural viral proteins (NSP1-6) [14]. Outer capsid proteins VP7 and VP4, which are each independent neutralization and protective antigens, define VP7 or G serotype/genotype and VP4 or P serotype/genotype, respectively [14]. A total of 15 G genotypes and 27 P genotypes have been detected in humans and animals combined [14, 42]. At least nine G (G1, G2, G3, G6, G7, G8, G10, G11 and G15) and six P genotypes (P[1], P[5], P[11], P[14], P[17] and P[21]) have been detected in cattle thus far. Among them, G genotypes G6, G8 and G10, and P genotypes P[1], P[5], and P[11] are of epizootiologic importance worldwide [11, 20, 49, 52, 53, 62]. Bovine rotaviruses detected in early studies have played a significant role in research and development of human rotavirus vaccines. For example, (i) US bovine rotavirus strain NCDV (G6P6[1]) (44) was the first human rotavirus vaccine which was evaluated extensively in clinical trials [58, 68, 69]; (ii) British bovine rotavirus strain UK (G6P7[5]) [6] is a parental strain of the human-bovine reassortant rotavirus vaccine [31, 45, 46], which is currently under development in India, China and Brazil [25, 37]; and (iii) US bovine rotavirus strain WC3 (G6P7[5]) is a parental strain of a human-bovine reassortant vaccine which was licensed in the US in 2006 as well as in other countries [25].
A variety of techniques have been utilized to measure rotavirus-specific serum antibody responses in cattle including a neutralization assay [7, 8, 10, 23, 39, 57, 61, 63, 70–74], ELISA [39, 48, 57, 72], an epitope-blocking immunoassay [63], a radioimmunoassay [1, 2] and an immunocytochemical staining assay [75]. The immunocytochemistry staining assay that uses Sf-9 insect cells infected with recombinant baculovirus expressing selected rotavirus proteins as immobilized antigens to detect protein-specific antibody responses has been well established to be rapid, simple, sensitive, and specific [33, 34, 75, 76] More importantly, in this assay, unlike a radioimmunoprecipitation assay and Western blot (immunoblot) assay, antibodies to complex, conformation-dependent antigenic epitopes on both VP4 and VP7 protective antigens can be measured [33, 34].
Previously, we reported homotypic and heterotypic serum IgG antibody responses to a total of 7 different NSP4 proteins in gnotobiotic calves infected orally or intraamniotically with bovine rotavirus [75]. In the present study, using the immunocytochemical staining assay involving a total of six different recombinant baculoviruses with each expressing one of the major bovine rotavirus VP7 (G6, G8 or G10) and VP4 (P6[1], P7[5] or P8[11]) serotypes, we quantified IgG antibodies to individual proteins in archival serum samples collected from a cohort of 31 calves in an attempt to analyze the frequency and patterns of rotavirus infections and the distribution of G and P types of infecting rotaviruses.
MATERIALS AND METHODS
Serum samples
The project was initiated in 1974 by Dr. Francis R. Abinanti of the University of Arizona, Department of Veterinary Science, in collaboration with Dr. Albert Z. Kapikian of the National Institutes of Health to study the epidemiology and natural history of bovine rotaviruses in calves. Sequential serum samples were obtained from 31 Holstein calves at approximately monthly intervals from 1 month to 12 months of age during the 1974–1975 season at the Arizona Dairy Company located in Higley, Arizona. The calves were given colostrum immediately after birth from the dam of each calf, and raised separately from their mothers. The serum samples sent to NIH were stored in a −20°C freezer until testing. These samples were evaluated for rotavirus infection by complement fixation test (CFT) [36] using the “O” agent and the bovine NCDV strain in the mid-1970s at NIH (unpublished data). A total of 309 serum samples were analyzed in the current study.
Construction of recombinant baculoviruses expressing rotavirus VP4 or VP7 protein
Recombinant baculoviruses with each expressing one of the major bovine rotavirus VP4 (P6[1], P7[5] and P8[11]) or VP7 (G6, G8 and G10) proteins (Table 1) were constructed according to the manufacturer’s instructions or as described elsewhere [76]. The expression of a rotavirus protein was confirmed by Western blot analysis against type-specific polyclonal antibodies to the rotavirus protein. High-titer virus stocks of the recombinant baculoviruses were propagated in Sf-9 insect cells, and their titers were determined by using a plaque assay according to the manufacturer’s instructions (Bac-N-Blue Transfection Kit, Invitrogen).
Table 1.
Recombinant baculoviruses used in this study
| Protein | Serotype [genotype] | Rotavirus strain | Reference |
|---|---|---|---|
| VP7 | G6 | UK | [76] |
| G8 | 69M | Present study | |
| G10 | B223 | Present study | |
|
| |||
| VP4 | P7[5] | UK | [76] |
| P6[1] | NCDV | [76] | |
| P8[11] | B223 | Present study | |
Note: Expression vector: pBlueBac 4.5
Immunocytochemistry (Sf-9 insect cell staining) assay
Titers of serum IgG antibodies to each of the recombinant rotavirus VP4 and VP7 proteins were determined using an Sf-9 insect cell staining assay as previously described [33, 34, 75, 76]. Briefly, Sf-9 insect cells were infected with baculovirus expressing individual rotavirus proteins at an MOI of 0.1 and seeded onto 96-well tissue culture plates (Costar, Corning Inc., NY), and the plates were incubated for 72 h at 28°C. The culture medium was then removed and plates were air-dried in a biosafety cabinet at room temperature for 1 h. Cells were then fixed with 10% formalin (Sigma, St. Louis) for 30 min and permeabilized with 1% Triton X-100 (Sigma) in TNC (10 mM Tris, 100 mM NaCl, 1 mM CaCl2 [pH 7.4]) for 2 min. After two washes with L-15 medium, serial 2-fold dilutions of serum samples (starting at 1:50) were added to the plates. The plates were incubated for 2 h at 37°C and then washed twice with L-15 medium. For the detection of IgG antibodies, horseradish peroxidase-labeled goat anti-bovine IgG (H+L; Kirkegaard & Perry Laboratories, Inc.) was added as a detector antibody at a 1:3000 dilution in L-15 medium. The plates were incubated for 1 h at 37°C and then washed twice with L-15 medium. Bound antibodies were visualized with AEC substrate (3-amino-9ethyl-carbazole; Sigma). The antibody titer was defined as the reciprocal of the highest dilution at which any positive cell staining could be detected under the microscope at 100× magnification, as described elsewhere [33, 34, 75, 76].
Seroresponse Indexes
Protein-specific IgG antibody titers against each VP7 (G) or VP4 (P) type were converted to a seroresponse index representing the fold increase in antibody titer in paired sera that were collected sequentially in a given period of one or two months from each calf. A four-fold or greater seroresponse index to the specific antigen (e.g., G6 VP7 protein) that was detected in a given paired sera was considered to indicate that the calf (from which the paired sera were derived) was infected with a rotavirus bearing that specific antigen (e.g., G6 specificity) during that period of one or two months.
Statistical analysis
Geometric mean serum IgG antibody titers to each VP7 or VP4 protein in calves which experienced the first seroresponse either before or ≥ 4 months of age were analyzed using Student’s t test with SPSS 14.0 package (SPSS Inc). Statistical significance was assessed at P<0.05.
RESULTS
Frequency and patterns of serum IgG antibody seroresponse to protective antigens VP7 and VP4
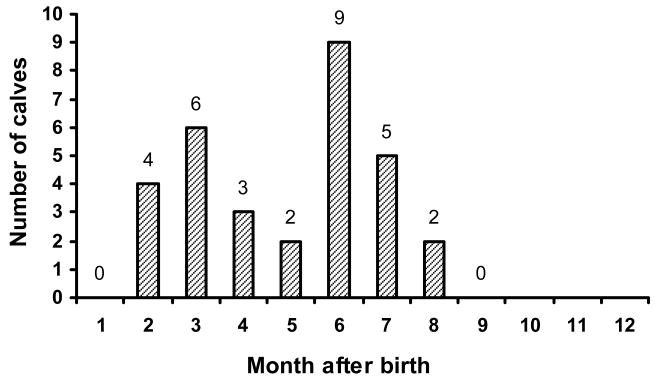
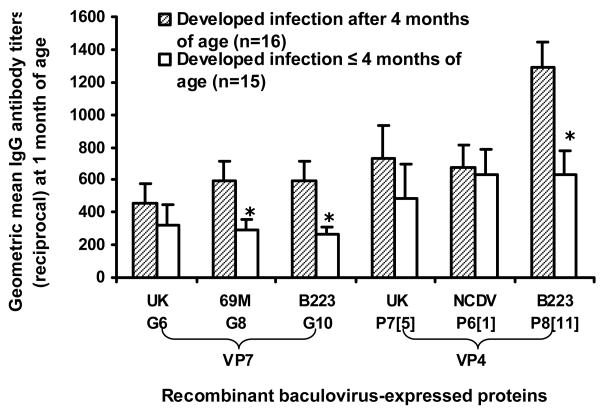
Each of 31 calves experienced at least one rotavirus infection as determined by a 4-fold or greater G- and/or P-serotype-specific antibody response during the period of 12 months. Twenty-nine of the 31 (93.6%) calves had at least one rotavirus infection as determined by CFT (data not shown). The first G- and/or P-serotype-specific seroresponse was detected between 2 and 8 months of age (Fig. 1): a seroresponse was not detected after 9 months of age. Since serum samples collected at birth were not available, we could not determine seroresponse in calves during the first month of life. The influence of preexisting antibodies at 1 month of age on the timing of a seroresponse was variable for different serotypes (Fig. 2). For example, calves that experienced a seroresponse between 2 and less than 4 months of age to 69M (G8), B223 (G10) or B223 (P8[11]) had significantly lower IgG antibody titers at 1 month of age whereas those that experienced a seroresponse between 2 and less than 4 months of age to UK (G6), UK (P7[5]) or NCDV (P6[1]) did not (Fig. 2).
Figure 1.
Age of calves at which the first rotavirus-specific seroresponse was detected.
Figure 2.
Relationship of preexisting antibody titer at 1 month of age with the time of developing serologic evidence of infection to various proteins. The asterisk (*) indicates a significant difference between two groups (P<0.05, independent t test). The error bars represent standard errors of the means.
Of note was the finding that VP4- and VP7-specific seroresponses were not always elicited concurrently in calves following infection. Only 25.8% of calves developed concurrent VP4- and VP7-specific seroresponses, whereas 45.2% of calves had a VP4-specific seroresponse prior to that to VP7, and 29.0% of calves exhibited VP7-specific seroresponse prior to that to VP4.
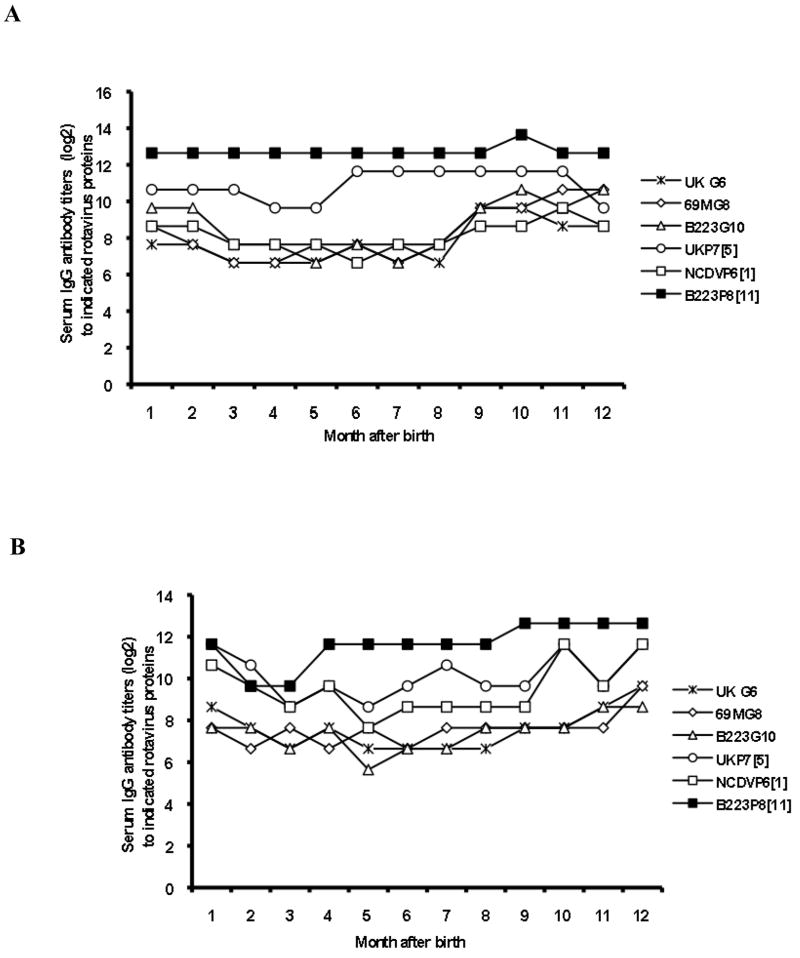
Figure 3 represents longitudinal serum IgG antibody profiles to 3 VP7 proteins (UK G6, 69M G8 and B223 G10) and 3 VP4 proteins (UK P7[5], NCDV P6[1] and B223 P8[11]) of two calves: one that experienced a single rotavirus infection and another (case #2 in Table 3) that experienced repeated rotavirus infections. It was noteworthy that existing antibody levels at one month of age varied depending upon G and/or P type(s). Both calves exhibited a decline in antibody titers to selected G and/or P proteins during the first 5 months of age, which may have reflected the decrease of maternally-derived antibodies in the calves to such proteins. One calf developed seroresponse to P7[6] VP4 at 6 months of age as well as seroresponse to both G6 and G8 VP7s at 9 months of age (Fig. 3A). Another calf demonstrated the 1st seroresponse to P8[11] VP4 at 4 months of age and the 2nd seroresponse to P6[1] and P7[5] VP4s at 10 months of age. Of note was the finding that the 1st seroresponse to VP7 (G8) was not detected in this calf until 12 months of age (Fig. 3B).
Figure 3.
Longitudinal serum IgG antibody profiles to 3 VP7 proteins (UK G6, 69M G8 and B223 G10) and 3 VP4 proteins (NCDV P6[1], UK P7[5] and B223 P8[11]) of two calves: one that experienced a single rotavirus infection (A) and another (case #2 in Table 3) that experienced repeated rotavirus infections (B).
Table 3.
Sequential rotavirus infection cases deduced from analyses of protein-specific antibody responses or detected by CFT.
| Case No. | 1st seroconversion detected by indicated assay (at indicated month) |
2nd seroconversion detected by indicated assay (at indicated month) |
||||||
|---|---|---|---|---|---|---|---|---|
| CFT versus |
Immunostaining assay versus |
CFT versus |
Immunostaining assay versus |
|||||
| “O” agent | NCDV | G protein | P protein | “O” agent | NCDV | G protein | P protein | |
| 1 | + (7) | - | G8 (9) | P8[11] (5) | - | - | ND* | P6[1] (6) |
| 2 | + (6) | - | G8 (12) | P8[] (4) | - | + (8) | ND | P6[1], P7[5] (10) |
| 3 | + (8) | +(8) | G8 (5) | P8[11] (7) | - | - | G6 (9) | P6[1] (9) |
| 4 | + (3) | + (2) | G6 (3) | P8[11] (2) | + (6) | + (6) | G6, G8 (6) | ND |
| 5 | + (5) | + (6) | G8 (2) | P7[5] (2) | - | + (9) | G10 (8) | P7[5] (5) |
| 6 | + (6) | - | G8 (8) | P6[1] (3) | - | - | ND | P8[11] (6) |
| 7 | + (5) | - | G10 (9) | P7[5] (8) | - | + (8) | ND | P8[11] (9) |
| 8 | + (6) | + (6) | G6, G8 (6) | P6[1], P8[11] (7) | + (9) | - | G6, G8, G10 (8) | P6[1], P7[5] (8) |
| 9 | + (6) | + (6) | G8 (4) | P7[5] (5) | + (10) | + (10) | ND | P6[1] (6) |
| 10 | + (5) | - | G8 (7) | P7[5] (7) | + (7) | - | ND | P6[1] (9) |
| 11 | + (2) | - | G8 (4) | P6[1], P7[5] (6) | + (6) | + (6) | ND | P8[11] (7) |
| 12 | + (9) | - | G8 (2) | P7[5] (8) | + (10) | - | ND | P6[1], P7[5] (10) |
| 13 | + (8) | - | G10 (4) | P8[11] (6) | - | + (9) | ND | P6[1], P7[5] (7) |
| 14 | + (7) | - | G6 (3) | P6[1] (4) | - | - | G6 (7) | ND |
| 15 | + (9) | + (9) | G8 (3) | P6[1], P8[11] (3) | - | - | G8 (7) | P6[1] (9) |
| 16 | + (9) | + (9) | G6, G10 (8) | P6[1], P7[5] (7) | - | - | ND | P6[1], P7[5] (8) |
| 17 | + (7) | + (7) | G6 (7) | P6[1] (7) | + (9) | + (9) | G8 (8) | ND |
| 18 | + (7) | - | G8 (5) | P8[11] (3) | - | - | ND | P6[1] (7) |
| 19 | + (2) | + (2) | G8 (5) | P7[5] (2) | + (6) | + (6) | G10 (6) | ND |
| 20 | + (6) | - | G10 (4) | P8[11] (6) | - | - | G8 (7) | ND |
| 21 | + (5) | - | G6, G8, G10 (8) | P6[1] (6) | - | + (8) | ND | ND |
Frequency of rotavirus infections and distribution of G and P types of infecting rotaviruses
Analyses of serum samples showed that seven (22.6%) of 31 calves experienced a single rotavirus infection whereas 14 (45.2%) had multiple P type infections; 7 (22.6%) had multiple G type infections; and 3 (9.7%) had mixed G and P type infections. Analyses of protein-specific antibody responses indicated that the G8 serotype (35.5%) infected calves most frequently followed by G6 (19.4%) and G10 (12.9%); whereas 32.3% of calves experienced mixed G type infections (Table 2). Regarding the distribution of P types, an IgG response to P6[1] was detected in 25.8% of samples followed by P7[5] (12.9%) and P8[11] (6.5%), whereas 54.8% of samples demonstrated responses to multiple P types (Table 2). Some of the mixed G and/or P type seroresponses may have reflected a broadening of the immune response to cross-reactive epitopes of the infecting single rotavirus strain. The most frequent G-P combination of infecting rotavirus was G8P6[1] followed by G8P7[5], G8P8[11] and G6P6[1]. Thus, our retrospective analyses of serum samples showed that G8 and P6[1] were the predominant bovine rotavirus types infecting cattle during the 1974–1975 season, in Higley, Arizona.
Table 2.
Distribution of G and P serotypes of infecting bovine rotaviruses deduced from analyses of serum IgG antibody responses.
| Serotype [genotype] | Number (%) of positive calves | |
|---|---|---|
| G type | G6 | 6 (19.4) |
| G8 | 11 (35.5) | |
| G10 | 4 (12.9) | |
| Mixed G | 10 (32.3) | |
|
| ||
| P type | P7[5] | 4 (12.9) |
| P6[1] | 8 (25.8) | |
| P8[11] | 2 (6.5) | |
| Mixed P | 17 (54.8) | |
Repeated rotavirus infections
Twenty of 31 (64.5%) calves experienced repeated rotavirus infections as determined by the immunocytochemical staining assay; 12 of the 20 were detected by the CFT also and in addition, one infection was detected by the CFT only (Table 3). Of note was the finding that in each of the 20 calves, the primary rotavirus infection was identified by both G type- and P type-specific seroresponses to the infecting rotavirus(es), whereas only four of the 20 (20.0%) 2nd rotavirus infections were identified by both G type- and P type-specific seroresponses. The distribution of seroresponses among the 11 calves that did not respond to both VP7 and VP4 was as follows: 5 developed a G type-specific seroresponse and 11 a P type-specific seroresponse. These varying patterns of 2nd seroresponses may reflect the influence of relative concentrations of VP7- and/or VP4-specific antibodies present at the time of second rotavirus infection(s) in calves that were induced by the primary infections. It is of interest that each of the 21 initial seroresponses was detected by the CFT using the “O” agent whereas only 9 of the 21 responses were detectable by using the NCDV strain (Table 3). However, eight and 9 of the 2nd seroresponses were detected by using the “O” agent and the NCDV strain, respectively. Recently, the “O” agent was shown to bear a G8P[1] specificity [60], which may, in part, be associated with the observed efficient detectability of the 1st seroresponse with the “O” agent; 14 of the 21 responses were deduced to be caused by the G8 or G8 plus other G types.
Only two of the 8 (25%) 2nd G-type seroresponses and two of the 15 (13.3%) 2nd P-type seroresponses were shown to be caused by the same G type and P type, respectively. These percentages were lower than those caused by the different G type and P type (X2 = 3.96, P=0.047), suggesting that protection against rotavirus reinfection was primarily homotypic.
DISCUSSION
In the present study, we had a rare opportunity to analyze rotavirus-specific antibodies in archival serum samples (n = 309) collected monthly from one month to one year of age from a cohort of 31 calves during the 1974–1975 season in Higley, Arizona. This was possible because such serum samples, which were sent from the University of Arizona, College of Agriculture to the NIH in the mid-1970s, were still available after storage at −20°C. Although the bovine rotavirus NCDV strain was one of the earliest group A rotaviruses that was cell culture-adapted and characterized [44], it was not possible to routinely identify G and P types of field bovine isolates until the mid-1990s when Gouvea et al. [27, 28] reported the development of a PCR typing methodology for bovine rotavirus G and P genotypes. Bovine rotavirus strain surveillance studies using such a methodology have established that G genotypes G6, G8 and G10 and P genotypes P[1], P[5] and P[11] are of epizootiologic importance worldwide. In the present study, we generated baculovirus recombinants expressing each of the three major G genotypes (G6, G8 and G10) and P genotypes (P[1], P[5] and P[11]) and, by using these recombinants in immunocytochemistry assays analyzed IgG antibody responses to each protein in the 309 calf serum samples from Arizona.
Although each of the 31 calves experienced at least one rotavirus infection as determined by a 4-fold or greater G- or P-serotype-specific antibody response during the 12 months period, it was unexpected to find that only 7 (22.6%) of the calves were infected with a single rotavirus strain. The mixed infection rate of 77.4% was considerably greater than the rate of ≤48.5% reported previously [3, 19, 35, 54, 62, 64]. Although the identification of rotavirus infections can be detected by examination of stools and/or by serologic responses, in general, the latter are more efficient. In one study [67], a serologic assay identified 77.0% of rotavirus infections in a cohort of 200 children monitored from birth to two years of age whereas analysis of shedding of virus in stools identified only 56% of rotavirus infections in the same group of children. Thus, it was not surprising that we identified serologic evidence of rotavirus infection in all of the 31 calves which might have experienced either a symptomatic or an asymptomatic infection. On the other hand, both RT-PCR genotyping and/or monoclonal antibody-based ELISA that were utilized in published reports [18–22, 24, 27, 28, 32, 54] analyzed only a single stool sample from each calf that was diarrheic.
It is conceivable that some of the mixed G and/or P type seroresponses identified in this study were not caused by actual G and/or P type mixed infections but rather reflected a broadening of the immune response after repeated infections [9, 10, 26, 61, 63, 67]. A limitation of this study was that since the calf stool samples were not available, the detection and genotyping of infecting rotaviruses in stools were not performed. Such analysis would have validated the designation of repeated infections based on seroresponses to additional serotypes. It is noteworthy that (i) in contrast to the high rate of a 2nd seroresponse to VP4 proteins (55%), such a response to VP7 proteins (25%) was significantly lower (X2 = 6.45, P=0.04); and (ii) the rate of seroresponse to multiple P types (54.8%) was significantly higher than that to multiple G types (32.3%) (X2 = 7.33, P=0.03). These findings may reflect the greater cross-reactivity of VP4 proteins when compared to VP7 proteins [12, 13, 50]. It is interesting to note that although several monoclonal antibody-based G-serotyping ELISA kits are commercially available, none is available for P-serotyping, likely because monoclonal antibodies to the VP4 are, in general, broadly P-type cross-reactive [12, 13, 50].
Of note was the finding that calves that experienced their first seroresponse between 2 and <4 months of age had a significantly lower antibody titer to certain serotypes at 1 month of age than those that developed a seroresponse later (Fig. 2), suggesting that maternal antibody might play a role in delaying primary infections and/or the development of antibody responses, as observed in gnotobiotic pigs [30, 51]. The transfer of circulating maternal serum IgG antibody (which is originally derived from colostrum) to the gastrointestinal tract in young calves has been well documented [4, 5, 16, 40]. Furthermore, the decrease in clinical signs or the protection from rotavirus infections associated with maternally derived high levels of serum IgG antibodies in neonatal calves have been reported previously [4, 5, 56].
Another interesting finding was that VP4- and VP7-specific seroresponses were not always elicited concurrently in rotavirus-infected calves. In addition, in some calves, a 2nd seroresponse was detected only for a G type(s) or for a P type(s) only. This may be a function of G or P type-specific antibodies in the calf small intestine at the time of rotavirus infection, which were derived from dams via colostrum or induced by primary rotavirus infections [4, 5, 30, 40].
Although the virus bearing a G6P6[1] specificity is used in most commercial bovine vaccines [3, 66], the most commonly detected bovine rotavirus genotype in many countries appears to be G6P[5] [3, 18, 19, 24, 35, 54, 62, 64]. In this retrospective analysis, serotype G8 was shown to be the predominant bovine rotavirus G type in the Higley, Arizona area during the 1974–1975 season. Serotype G8 virus was first recovered from a child with gastroenteritis in Indonesia in 1984 [29, 43]; the first bovine G8 strain was isolated in Scotland in 1984 also [47, 62]; later it was detected in North America and other countries [18, 19, 41, 49, 52, 65]; and appears to be epidemiologically important today in parts of Africa [59]. Various reports [18, 24, 49, 62] appear to indicate that G8 is one of the less common serotypes among bovine rotaviruses, however, the G8 has been reported to be the predominant G type in cattle in some parts of the world during certain periods of time [15, 17, 19]. Interestingly, retrospective analyses reported in 1995 demonstrated [41] that the bovine Cody strain (NCDV-Cody) isolated in Nebraska in 1968, which was thought initially to be the virulent counterpart for the attenuated NCDV strain (NCDV-Lincoln, G6P[1]), bore a G8P[1] specificity. Thus, that report and our current data indicate that the bovine G8 serotype has been circulating among cattle in the USA for more than 3 decades.
Our analyses of VP7- and VP4-specific antibody responses in calves indicated that (i) repeated infections were less likely to be caused by the G and/or P types than those caused by primary infections (X2 = 3.96, P=0.047); and (ii) protection against rotavirus reinfection tended to be serotype specific. Similar trend (i.e., second infections were more likely to be caused by another G type) was also observed in a cohort study involving 200 Mexican infants monitored weekly for rotavirus excretion and diarrhea from birth to age 2 years [67]. In a recent large scale, double-blind, placebo-controlled trial of more than 63,000 infants enrolled in 11 Latin American countries and Finland, the monovalent G1P[8] vaccine (RIX4414) proved to be strongly efficacious in preventing rotavirus diarrhea of any severity caused by the G- and/or P-type-homotypic viruses (i.e., G1P[8], G3P[8], G4P[8], and G9P[8]), however, efficacy against the G-and P-type heterotypic virus (i.e., G2P[4]) was not significant [55]. Although there is still considerable disagreement on the role and importance of serotype-specific immunity in protection against rotavirus-caused illness [38], our data support the strategy of the administration of a multivalent vaccine and multiple vaccinations in order to elicit broader protection against prevailing serotypes in humans [67].
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Disease, National Institutes of Health, USA. There is no conflict of interest to declare.
References
- 1.Acres SD, Babiuk LA. Studies on rotaviral antibody in bovine serum and lacteal secretions, using radioimmunoassay. J Am Vet Med Assoc. 1978;173:555–559. [PubMed] [Google Scholar]
- 2.Babiuk LA, Acres SD, Rouse BT. Solid-phase radioimmunoassay for detecting bovine (neonatal calf diarrhea) rotavirus antibody. J Clin Microbiol. 1977;6:10–15. doi: 10.1128/jcm.6.1.10-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreiros MA, Alfieri AF, Medici KC, et al. G and P genotypes of group A rotavirus from diarrhoeic calves born to cows vaccinated against the NCDV (P[1], G6) rotavirus strain. J Vet Med B Infect Dis Vet Public Health. 2004;51:104–109. doi: 10.1111/j.1439-0450.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 4.Besser TE, Gay CC, McGuire TC, et al. Passive immunity to bovine rotavirus infection associated with transfer of serum antibody into the intestinal lumen. J Virol. 1988;62:2238–2242. doi: 10.1128/jvi.62.7.2238-2242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besser TE, McGuire TC, Gay CC, et al. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J Virol. 1988;62:2234–2237. doi: 10.1128/jvi.62.7.2234-2237.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridger JC, Woode GN. Neonatal calf diarrhoea: identification of a reovirus-like (rotavirus) agent in faeces by immunofluorescence and immune electron microscopy. Br Vet J. 1975;131:528–535. [PubMed] [Google Scholar]
- 7.Bridger JC, Brown JF. Antigenic and pathogenic relationships of three bovine rotaviruses and a porcine rotavirus. J Gen Virol. 1984;65(Pt 7):1151–1158. doi: 10.1099/0022-1317-65-7-1151. [DOI] [PubMed] [Google Scholar]
- 8.Bridger JC, Oldham G. Avirulent rotavirus infections protect calves from disease with and without inducing high levels of neutralizing antibody. J Gen Virol. 1987;68(Pt 9):2311–2317. doi: 10.1099/0022-1317-68-9-2311. [DOI] [PubMed] [Google Scholar]
- 9.Brussow H, Walther I, Fryder V, et al. Cross-neutralizing antibodies induced by single serotype vaccination of cows with rotavirus. J Gen Virol. 1988;69(Pt 7):1647–1658. doi: 10.1099/0022-1317-69-7-1647. [DOI] [PubMed] [Google Scholar]
- 10.Brussow H, Eichhorn W, Rohwedder A, et al. Cattle develop neutralizing antibodies to rotavirus serotypes which could not be isolated from faeces of symptomatic calves. J Gen Virol. 1991;72(Pt 7):1559–1567. doi: 10.1099/0022-1317-72-7-1559. [DOI] [PubMed] [Google Scholar]
- 11.Chang KO, Parwani AV, Saif LJ. The characterization of VP7 (G type) and VP4 (P type) genes of bovine group A rotaviruses from field samples using RT-PCR and RFLP analysis. Arch Virol. 1996;141:1727–1739. doi: 10.1007/BF01718295. [DOI] [PubMed] [Google Scholar]
- 12.Coulson BS. Typing of human rotavirus VP4 by an enzyme immunoassay using monoclonal antibodies. J Clin Microbiol. 1993;31:1–8. doi: 10.1128/jcm.31.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulson BS. VP4 and VP7 typing using monoclonal antibodies. Arch Virol Suppl. 1996;12:113–118. doi: 10.1007/978-3-7091-6553-9_13. [DOI] [PubMed] [Google Scholar]
- 14.Estes MK, kapikian AZ. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus EE, editors. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1917–1974. [Google Scholar]
- 15.Falcone E, Tarantino M, Di Trani L, et al. Determination of bovine rotavirus G and P serotypes in italy by PCR. J Clin Microbiol. 1999;37:3879–3882. doi: 10.1128/jcm.37.12.3879-3882.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez FM, Conner ME, Hodgins DC, et al. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from cows immunized with recombinant SA11 rotavirus core-like particle (CLP) or virus-like particle (VLP) vaccines. Vaccine. 1998;16:507–516. doi: 10.1016/S0264-410X(97)80004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fodha I, Boumaiza A, Chouikha A, et al. Detection of group a rotavirus strains circulating in calves in Tunisia. J Vet Med B Infect Dis Vet Public Health. 2005;52:49–50. doi: 10.1111/j.1439-0450.2004.00810.x. [DOI] [PubMed] [Google Scholar]
- 18.Fukai K, Sakai T, Kamata H. Distribution of G serotypes and P genotypes of bovine group A rotavirus isolated in Japan. Aust Vet J. 1998;76:418–422. doi: 10.1111/j.1751-0813.1998.tb12393.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukai K, Sakai T, Hirose M, et al. Prevalence of calf diarrhea caused by bovine group A rotavirus carrying G serotype 8 specificity. Vet Microbiol. 1999;66:301–311. doi: 10.1016/s0378-1135(99)00021-8. [DOI] [PubMed] [Google Scholar]
- 20.Fukai K, Maeda Y, Fujimoto K, et al. Changes in the prevalence of rotavirus G and P types in diarrheic calves from the Kagoshima prefecture in Japan. Vet Microbiol. 2002;86:343–349. doi: 10.1016/s0378-1135(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 21.Fukai K, Saito T, Inoue K, et al. Molecular characterization of novel P[14], G8 bovine group A rotavirus, Sun9, isolated in Japan. Virus Res. 2004;105:101–106. doi: 10.1016/j.virusres.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Fukai K, Takahashi T, Tajima K, et al. Molecular characterization of a novel bovine group A rotavirus. Vet Microbiol. 2007;123:217–224. doi: 10.1016/j.vetmic.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganaba R, Belanger D, Dea S, et al. A seroepidemiological study of the importance in cow-calf pairs of respiratory and enteric viruses in beef operations from northwestern Quebec. Can J Vet Res. 1995;59:26–33. [PMC free article] [PubMed] [Google Scholar]
- 24.Garaicoechea L, Bok K, Jones LR, et al. Molecular characterization of bovine rotavirus circulating in beef and dairy herds in Argentina during a 10-year period (1994–2003) Vet Microbiol. 2006;118:1–11. doi: 10.1016/j.vetmic.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Glass RI, Parashar UD, Bresee JS, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–332. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 26.Gorrell RJ, Bishop RF. Homotypic and heterotypic serum neutralizing antibody response to rotavirus proteins following natural primary infection and reinfection in children. J Med Virol. 1999;57:204–211. [PubMed] [Google Scholar]
- 27.Gouvea V, Santos N, Timenetsky Mdo C. VP4 typing of bovine and porcine group A rotaviruses by PCR. J Clin Microbiol. 1994;32:1333–1337. doi: 10.1128/jcm.32.5.1333-1337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gouvea V, Santos N, Timenetsky Mdo C. Identification of bovine and porcine rotavirus G types by PCR. J Clin Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa A, Inouye S, Matsuno S, et al. Isolation of human rotaviruses with a distinct RNA electrophoretic pattern from Indonesia. Microbiol Immunol. 1984;28:719–722. doi: 10.1111/j.1348-0421.1984.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 30.Hodgins DC, Kang SY, deArriba L, et al. Effects of maternal antibodies on protection and development of antibody responses to human rotavirus in gnotobiotic pigs. J Virol. 1999;73:186–197. doi: 10.1128/jvi.73.1.186-197.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoshino Y, Jones RW, Ross J, et al. Construction and characterization of rhesus monkey rotavirus (MMU18006)- or bovine rotavirus (UK)-based serotype G5, G8, G9 or G10 single VP7 gene substitution reassortant candidate vaccines. Vaccine. 2003;21:3003–3010. doi: 10.1016/s0264-410x(03)00120-8. [DOI] [PubMed] [Google Scholar]
- 32.Hussein HA, Parwani AV, Rosen BI, et al. Detection of rotavirus serotypes G1, G2, G3, and G11 in feces of diarrheic calves by using polymerase chain reaction-derived cDNA probes. J Clin Microbiol. 1993;31:2491–2496. doi: 10.1128/jcm.31.9.2491-2496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida S, Feng N, Tang B, et al. Quantification of systemic and local immune responses to individual rotavirus proteins during rotavirus infection in mice. Journal of Clinical Microbiology. 1996;34:1694–1700. doi: 10.1128/jcm.34.7.1694-1700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida SI, Feng N, Gilbert JM, et al. Immune responses to individual rotavirus proteins following heterologous and homologous rotavirus infection in mice. Journal of Infectious Diseases. 1997;175:1317–1323. doi: 10.1086/516462. [DOI] [PubMed] [Google Scholar]
- 35.Ishizaki H, Sakai T, Shirahata T, et al. The distribution of G and P types within isolates of bovine rotavirus in Japan. Vet Microbiol. 1996;48:367–372. doi: 10.1016/0378-1135(95)00168-9. [DOI] [PubMed] [Google Scholar]
- 36.Kapikian AZ, Cline WL, Kim HW, et al. Antigenic relationships among five reovirus-like (RVL) agents by complement fixation (CF) and development of new substitute CF antigens for the human RVL agent of infantile gastroenteritis. Proc Soc Exp Biol Med. 1976;152:535–539. doi: 10.3181/00379727-152-39434. [DOI] [PubMed] [Google Scholar]
- 37.Kapikian AZ, Simonsen L, Vesikari T, et al. A hexavalent human rotavirus-bovine rotavirus (UK) reassortant vaccine designed for use in developing countries and delivered in a schedule with the potential to eliminate the risk of intussusception. J Infect Dis. 2005;192(Suppl 1):S22–29. doi: 10.1086/431510. [DOI] [PubMed] [Google Scholar]
- 38.Kapikian AZ, Hoshino Y. To serotype or not to serotype: that is still the question. J Infect Dis. 2007;195:611–614. doi: 10.1086/510862. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y, Nielsen PR, Hodgins D, et al. Lactogenic antibody responses in cows vaccinated with recombinant bovine rotavirus-like particles (VLPs) of two serotypes or inactivated bovine rotavirus vaccines. Vaccine. 2002;20:1248–1258. doi: 10.1016/s0264-410x(01)00404-2. [DOI] [PubMed] [Google Scholar]
- 40.Kohara J, Tsunemitsu H. Correlation between maternal serum antibodies and protection against bovine rotavirus diarrhea in calves. J Vet Med Sci. 2000;62:219–221. doi: 10.1292/jvms.62.219. [DOI] [PubMed] [Google Scholar]
- 41.Lu W, Duhamel GE, Hoshino Y, et al. Characterization of the bovine group A rotavirus strain neonatal calf diarrhea virus-Cody (NCDV-Cody) J Clin Microbiol. 1995;33:990–994. doi: 10.1128/jcm.33.4.990-994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martella V, Ciarlet M, Banyai K, et al. Identification of group A porcine rotavirus strains bearing a novel VP4 (P) Genotype in Italian swine herds. J Clin Microbiol. 2007;45:577–580. doi: 10.1128/JCM.02262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuno S, Hasegawa A, Mukoyama A, et al. A candidate for a new serotype of human rotavirus. J Virol. 1985;54:623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mebus CA, Kono M, Underdahl NR, et al. Cell culture propagation of neonatal calf diarrhea (scours) virus. Can Vet J. 1971;12:69–72. [PMC free article] [PubMed] [Google Scholar]
- 45.Midthun K, Greenberg HB, Hoshino Y, et al. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985;53:949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Midthun K, Hoshino Y, Kapikian AZ, et al. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986;24:822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ojeh CK, Snodgrass DR, Herring AJ. Evidence for serotypic variation among bovine rotaviruses. Arch Virol. 1984;79:161–171. doi: 10.1007/BF01310809. [DOI] [PubMed] [Google Scholar]
- 48.Ojeh CK, Tsunemitsu H, Simkins RA, et al. Development of a biotin-streptavidin-enhanced enzyme-linked immunosorbent assay which uses monoclonal antibodies for detection of group C rotaviruses. J Clin Microbiol. 1992;30:1667–1673. doi: 10.1128/jcm.30.7.1667-1673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada N, Matsumoto Y. Bovine rotavirus G and P types and sequence analysis of the VP7 gene of two G8 bovine rotaviruses from Japan. Vet Microbiol. 2002;84:297–305. doi: 10.1016/s0378-1135(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 50.Padilla-Noriega L, Werner-Eckert R, Mackow ER, et al. Serologic analysis of human rotavirus serotypes P1A and P2 by using monoclonal antibodies. J Clin Microbiol. 1993;31:622–628. doi: 10.1128/jcm.31.3.622-628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parreno V, Hodgins DC, de Arriba L, et al. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J Gen Virol. 1999;80(Pt 6):1417–1428. doi: 10.1099/0022-1317-80-6-1417. [DOI] [PubMed] [Google Scholar]
- 52.Parwani AV, Hussein HA, Rosen BI, et al. Characterization of field strains of group A bovine rotaviruses by using polymerase chain reaction-generated G and P type-specific cDNA probes. J Clin Microbiol. 1993;31:2010–2015. doi: 10.1128/jcm.31.8.2010-2015.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao CD, Gowda K, Reddy BS. Sequence analysis of VP4 and VP7 genes of nontypeable strains identifies a new pair of outer capsid proteins representing novel P and G genotypes in bovine rotaviruses. Virology. 2000;276:104–113. doi: 10.1006/viro.2000.0472. [DOI] [PubMed] [Google Scholar]
- 54.Reidy N, Lennon G, Fanning S, et al. Molecular characterisation and analysis of bovine rotavirus strains circulating in Ireland 2002–2004. Vet Microbiol. 2006;117:242–247. doi: 10.1016/j.vetmic.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-Palacios GM, Perez-Schael I, Velazquez R, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 56.Saif LJ, Redman DR, Smith KL, et al. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or nonimmunized cows. Infect Immun. 1983;41:1118–1131. doi: 10.1128/iai.41.3.1118-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saif LJ, Smith KL, Landmeier BJ, et al. Immune response of pregnant cows to bovine rotavirus immunization. Am J Vet Res. 1984;45:49–58. [PubMed] [Google Scholar]
- 58.Saif LJ, Rosen BI, Parwani AV. Animal Rotaviruses. In: Kapikian AZ, editor. Viral infections of the gastrointestinal tract. Marcel Dekker; New York: 1994. pp. 279–368. [Google Scholar]
- 59.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 60.Small C, Barro M, Brown TL, et al. Genome heterogeneity of SA11 rotavirus due to reassortment with “O” agent. Virology. 2007;359:415–424. doi: 10.1016/j.virol.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snodgrass DR, Ojeh CK, Campbell I, et al. Bovine rotavirus serotypes and their significance for immunization. J Clin Microbiol. 1984;20:342–346. doi: 10.1128/jcm.20.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snodgrass DR, Fitzgerald T, Campbell I, et al. Rotavirus serotypes 6 and 10 predominate in cattle. J Clin Microbiol. 1990;28:504–507. doi: 10.1128/jcm.28.3.504-507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snodgrass DR, Fitzgerald TA, Campbell I, et al. Homotypic and heterotypic serological responses to rotavirus neutralization epitopes in immunologically naive and experienced animals. J Clin Microbiol. 1991;29:2668–2672. doi: 10.1128/jcm.29.11.2668-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki Y, Sanekata T, Sato M, et al. Relative frequencies of G (VP7) and P (VP4) serotypes determined by polymerase chain reaction assays among Japanese bovine rotaviruses isolated in cell culture. J Clin Microbiol. 1993;31:3046–3049. doi: 10.1128/jcm.31.11.3046-3049.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taniguchi K, Urasawa T, Pongsuwanna Y, et al. Molecular and antigenic analyses of serotypes 8 and 10 of bovine rotaviruses in Thailand. J Gen Virol. 1991;72(Pt 12):2929–2937. doi: 10.1099/0022-1317-72-12-2929. [DOI] [PubMed] [Google Scholar]
- 66.Theil KW, McCloskey CM. Rotavirus shedding in feces of gnotobiotic calves orally inoculated with a commercial rotavirus-coronavirus vaccine. J Vet Diagn Invest. 1995;7:427–432. doi: 10.1177/104063879500700401. [DOI] [PubMed] [Google Scholar]
- 67.Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 68.Vesikari T, Isolauri E, Delem A, et al. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet. 1983;2:807–811. doi: 10.1016/s0140-6736(83)90734-1. [DOI] [PubMed] [Google Scholar]
- 69.Vesikari T. Clinical trials of live oral rotavirus vaccines: the Finnish experience. Vaccine. 1993;11:255–261. doi: 10.1016/0264-410x(93)90026-t. [DOI] [PubMed] [Google Scholar]
- 70.Woode GN, Kelso NE, Simpson TF, et al. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983;18:358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woode GN, Zheng SL, Rosen BI, et al. Protection between different serotypes of bovine rotavirus in gnotobiotic calves: specificity of serum antibody and coproantibody responses. J Clin Microbiol. 1987;25:1052–1058. doi: 10.1128/jcm.25.6.1052-1058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyatt RG, Mebus CA, Yolken RH, et al. Rotaviral immunity in gnotobiotic calves: heterologous resistance to human virus induced by bovine virus. Science. 1979;203:548–550. doi: 10.1126/science.216077. [DOI] [PubMed] [Google Scholar]
- 73.Wyatt RG, Kapikian AZ, Mebus CA. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983;18:505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Z, Hardy ME, Williams JD, et al. Immunodominant neutralizing antigens depend on the virus strain during a primary immune response in calves to bovine rotaviruses. Vet Microbiol. 1993;35:33–43. doi: 10.1016/0378-1135(93)90114-m. [DOI] [PubMed] [Google Scholar]
- 75.Yuan L, Honma S, Ishida S, et al. Species-specific but not genotype-specific primary and secondary isotype-specific NSP4 antibody responses in gnotobiotic calves and piglets infected with homologous host bovine (NSP4[A]) or porcine (NSP4[B]) rotavirus. Virology. 2004;330:92–104. doi: 10.1016/j.virol.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 76.Yuan L, Ishida S, Honma S, et al. Homotypic and heterotypic serum isotype-specific antibody responses to rotavirus nonstructural protein 4 and viral protein (VP) 4, VP6, and VP7 in infants who received selected live oral rotavirus vaccines. J Infect Dis. 2004;189:1833–1845. doi: 10.1086/383416. [DOI] [PubMed] [Google Scholar]