Fig. 3.
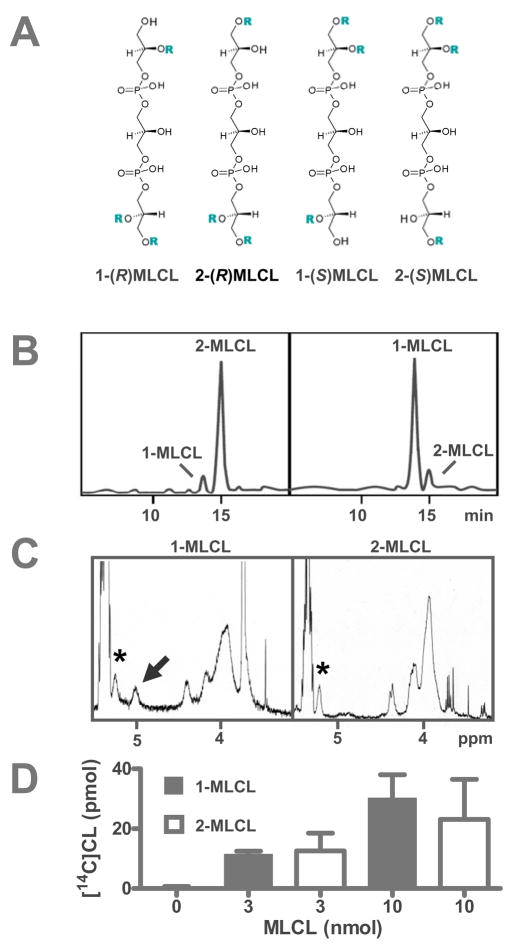
Both 1-MLCL and 2-MLCL are substrates of tafazzin. A, MLCL has four isomers with different positions of the acyl groups (R), which affects the chirality of the central carbon atom. B, MLCL isomers were separated by reversed-phase HPLC. Absorbance at 205 nm was plotted against retention time. The left chromatogram shows a commercial preparation of MLCL. The right chromatogram shows MLCL generated by treatment of bovine heart CL with Rhizopus arrhizus. C, 1H-NMR spectra showed that 1-MLCL contained two >CH-O-CO groups, one in the phosphatidyl moiety (asterisk) and one in the 1-lysophophatidyl moiety (arrow). 2-MLCL contained only one >CH-O-CO group in the phosphatidyl moiety (asterisk). Line assignments were made according to 1H-NMR data of the analogous LPC isomers [39] and NMR simulation. D, Both 1-MLCL and 2-MLCL reacted with purified tafazzin (MBP-TAZ). MBP-TAZ (2.5 μg) was incubated with the isomers and 2 nmol 16:0-[14C]18:2-PC (0.1 μCi) in 0.2 mL buffer for 30 minutes. Results are expressed as mean ± range of duplicate determinations.