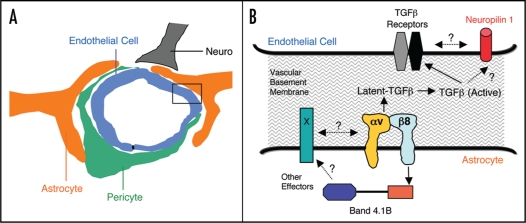
Figure 1.
A Model of αvβ8 integrin-mediated adhesion and signaling pathways in neurovascular units. (A) The multicellular composition of a central nervous system neurovascular unit, comprised of a capillary endothelial cell and pericyte, as well as neurons and astrocytes that adhere to extracellular matrix proteins within intervening vascular basement membranes. (B) A more detailed view of the boxed area in (A) showing the astrocyte-endothelial cell interface that is coupled, in part, by the αvβ8 integrin-TGFβ adhesion and signaling axis. αvβ8 integrin on the astrocyte binds to latent TGFβ's in the vascular basement membrane and induces TGFβ activation, resulting in liberation of TGFβ from the extracellular matrix. TGFβ signaling pathways in endothelial cells are likely regulated by canonical TGFβ receptors, but may also involve cross talk with Neuropilin 1. In astrocytes, αvβ8 integrin also interacts with Band 4.1B, a cytoskeletal adaptor protein, which may link this integrin with other intracellular and cell surface signaling effectors. Various molecular genetic data reveal that perturbation of normal astrocyte-endothelial cell coupling, via ablation of genes encoding the αvβ8 integrin or TGFβ signaling components, leads to abnormal neurovascular development and premature death.