Figure 2.
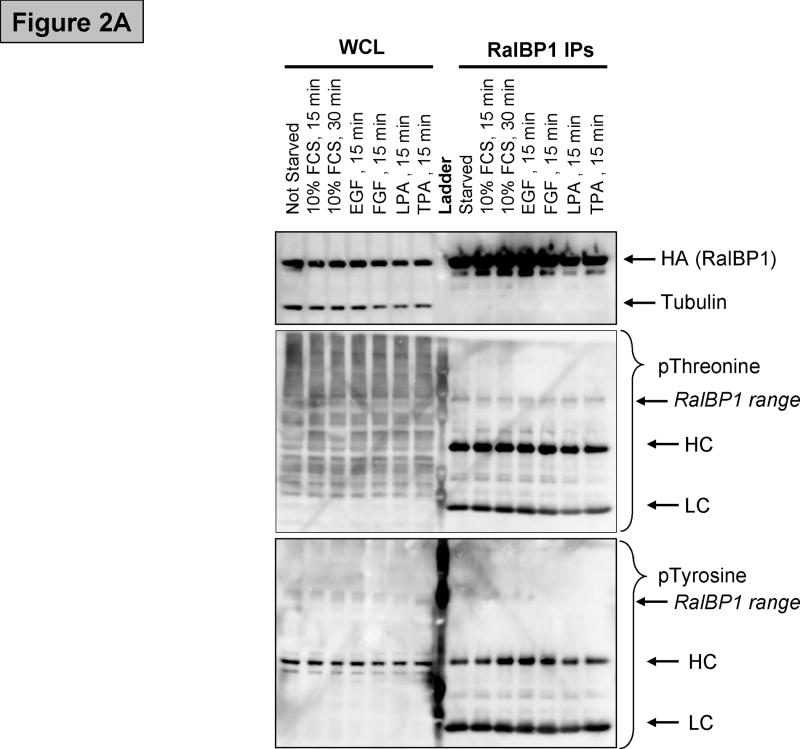
(A) Western blot showing RalBP1 immunoprecipitation (IP) of cells transfected with RalBP1-HA, serum-starved for 24 hours and stimulated as indicated. Analysis of whole cell lysates (WCL) indicates that equal amounts of RalBP1-HA were expressed in cells. Comparable levels of RalBP1-HA were precipitated, as indicated by HA-western blot. No differences in phosphorylation were detected by phosphor-Threonine or Tyrosine specific antibodies. Heavy and light chains (HL and LC) are marked accordingly. (B) Primary sequence of RalBP1 showing peptides detected in mass spectroscopic analysis (yellow). 406 of 655 amino acids, 62%, were covered. Fourteen sites were detected (bold). Two sites were ambiguous. It was unclear whether S116 or S118 was phosphorylated or whether it was S252 or T253 that was modified. 29 of the 41 Serine residues, 17 of 26 Threonine and 8 of 12 Tyrosine residues were covered.