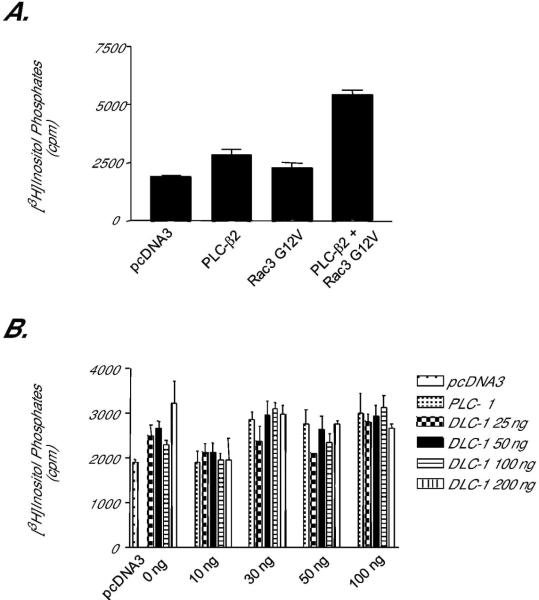
Figure 7.
DLC-1 does not stimulate phosphoinositide hydrolysis activity of PLC-δ1. Cos-7 cells were transiently transfected with vectors encoding various PLC isoforms and known or putative activators. Intracellular inositol phosphate accumulation was quantified as described in Methods. (A) PLC-β2 phospholipid hydrolysis activity is activated by constitutively activated Rac3. To verify the assay conditions, we coexpressed PLC-β2 with Rac3 G12V, an established activator of PLC-β2, and quantified [3H]inositol phosphate accumulation. (B) PLC-δ1 activity is not stimulated by DLC-1. Zero toδ100 ng of expression vector encoding PLC-δ1 was co-transfected with 0, 25, 50, 100, or 200 ng of DNA encoding human DLC-1, and the accumulation of [3H]inositol phosphates was quantified. Data shown are the mean ± SD of triplicate determinations and are representative of results obtained in two independent experiments.