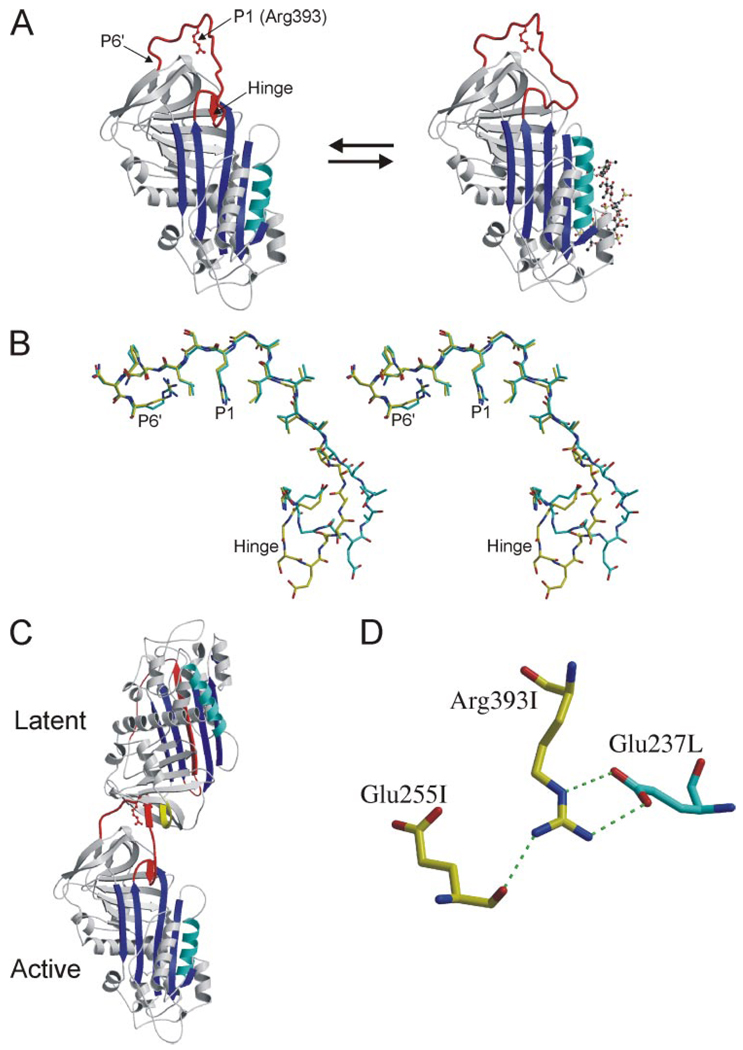
FIGURE 1. RCL conformation and contacts from crystals of the AT heterodimer.
A, The structures of native (left) and pentasaccharide-bound AT (right), solved in the context of the heterodimer with a latent AT counterpart, are shown as ribbon diagrams with the RCL in red, β-sheet A in blue, and heparin-binding helix D in cyan (the pentasaccharide is depicted as a ball-and-stick, and P1 Arg-393 is shown as a red ball-and-stick). The hinge region of the RCL (indicated) is partially incorporated as strand 4 of β-sheet A in the native state and is expelled upon heparin binding. Although the conformation of the hinge region was different in the two structures, the rest of the RCL, constrained by contacts with latent AT, did not differ. B, stereo diagram of the RCL of native (yellow) and pentasaccharide-bound (cyan) ATs (from P17 in the hinge region to the C-terminal end of the RCL at P6′) illustrates the identical RCL conformations from P8 to P6′. C, ribbon diagram of the AT heterodimer between active AT (bottom) and the latent counterpart (top) shows the intimate interaction involving the RCL of the active monomer with s2C (yellow) of the latent monomer. D, one of the principal contacts between the active (I, yellow) and latent (L, cyan) monomers involves the P1 residue (Arg-393) of active AT with Glu-237 from latent AT. The only intramolecular contact involving the side chain of the P1 residue is a hydrogen bond with the main chain of Glu-255.