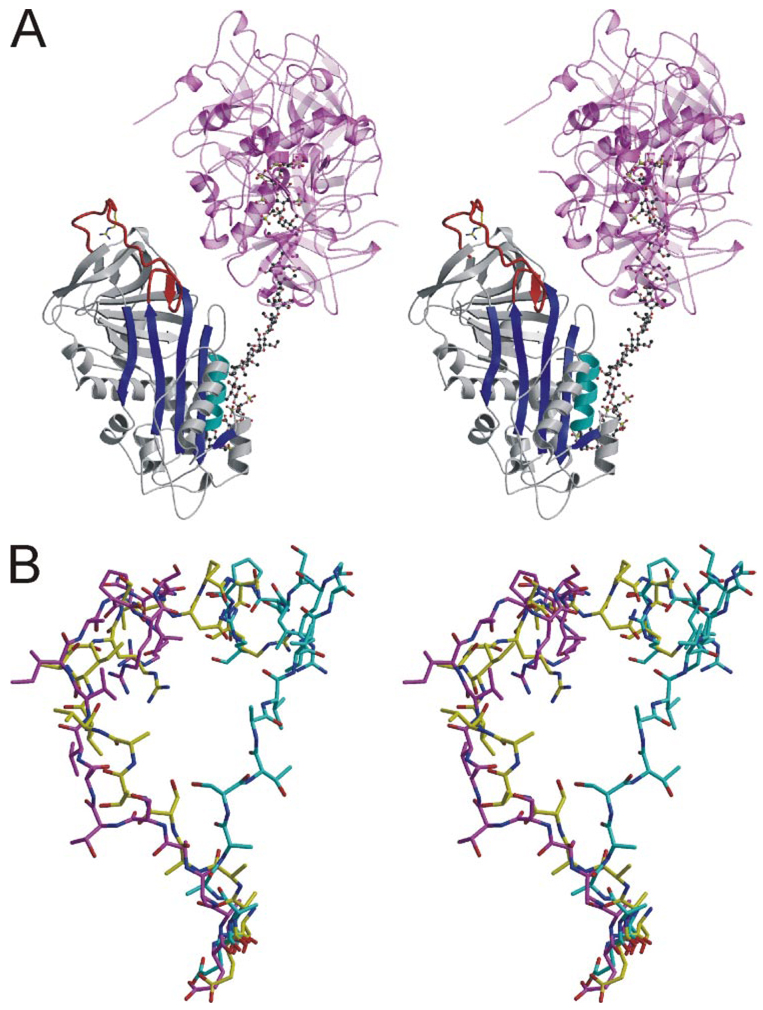
FIGURE 4. Structure of V317C/T401C AT from a different crystal form reveals similar RCL conformation and contacts.
A, stereo view of a ribbon diagram of the AT (colored as before), heparin (ball-and-stick), and S195A thrombin (semitransparent magenta and pink) complex. The RCL is clearly in a native-like conformation despite the presence of bound heparin. Two thrombin molecules bind to either side of the same site on heparin, and their active sites are occupied by the C terminus of adjacent light chains. B, the RCLs of native AT from the heterodimer (cyan) is compared with those of the V317C/T401C variant in the monomeric form (yellow) and in its nonproductive thrombin complex (magenta). The RCLs are shown from hinge region residue P14 (bottom) to P6′ (top).