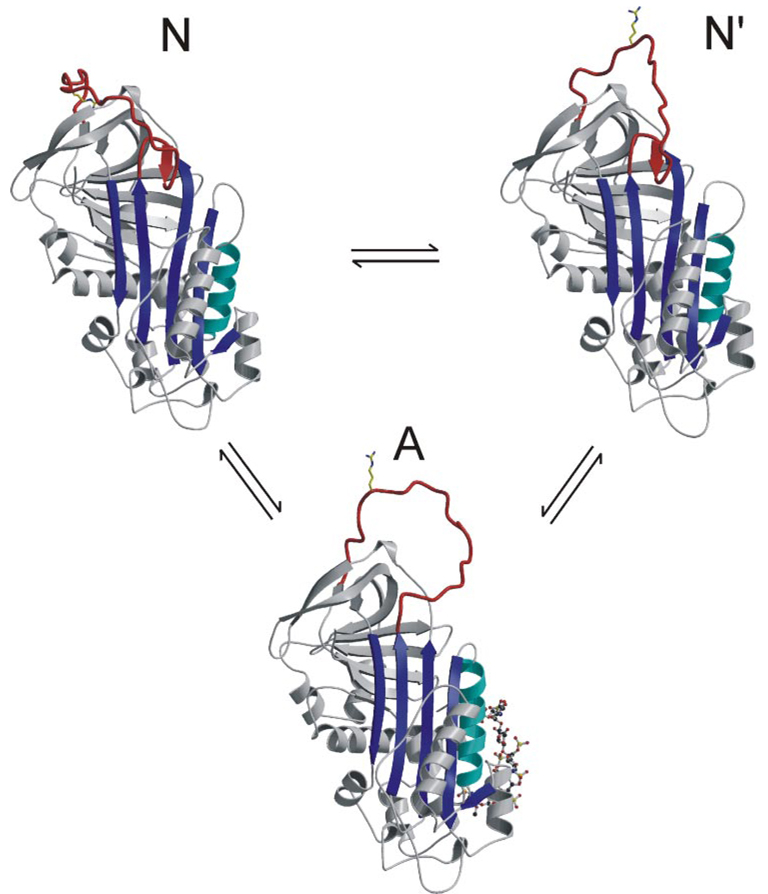
FIGURE 5. Equilibrium model for the conformational states of AT.
Similar to the revised model proposed by Chuang et al. (9), our data support a two-state conformational equilibrium for AT in the absence of heparin. Ribbon diagrams are given to represent the one activated and two native states. State N is the monomeric AT structure presented here with its RCL held close against the body of AT and the P1 side chain sequestered in the acidic pocked used as an exosite for factor Xa binding. This state would be nonreactive toward proteases but is in rapid equilibrium with state N′ (based on the structure of heterodimeric native AT), where there are fewer contacts to constrain the RCL and the P1 residue is free to interact with proteases. Activation of AT by the pentasaccharide results ultimately in the expulsion of the hinge and the liberation of the entire RCL. Monomer A is based on the structure of AT in complex with the pentasaccharide and S195A factor Xa (23).