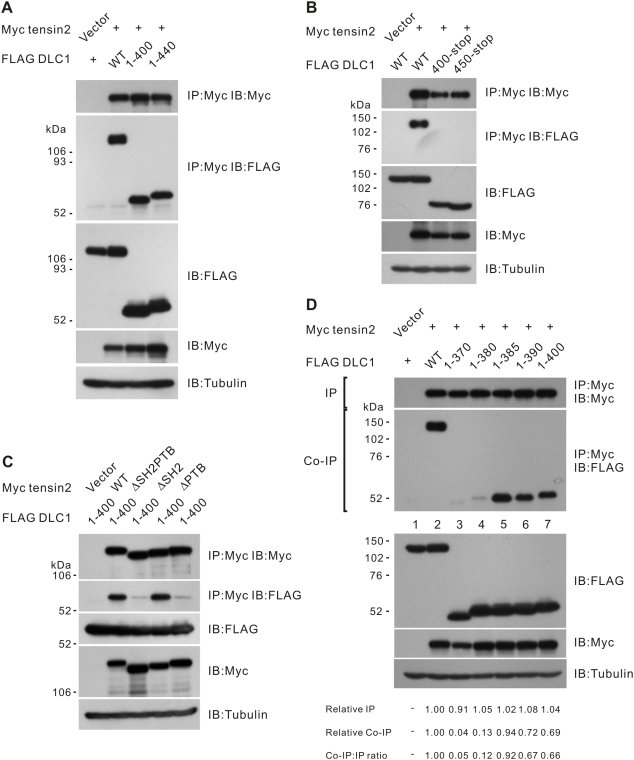
Figure 2. Mapping the tensin2 PTB binding site in DLC1.
(A) The N-terminus of DLC1 was sufficient for interaction with tensin2. HEK293T cells were transfected with Myc-tagged tensin2 fragment and FLAG-tagged DLC1 constructs as indicated. Cleared cell lysates were incubated with anti-Myc antibody to immunoprecipitate tensin2. DLC1 in the precipitates was detected by immunoblotting with anti-FLAG antibody. (B) The C-terminus of DLC1 was unable to bind tensin2. Myc-tagged tensin2 was co-transfected with two FLAG-tagged DLC1 C-terminus fragments as indicated. These DLC1 C-terminus fragments could not be co-immunoprecipitated with tensin2. (C) N-terminal fragment 1–400 was involved in tensin2 PTB binding. FLAG-tagged DLC1 1–400 could be co-immunoprecipitated with tensin2. Removal of the tensin2 SH2 domain did not affect the affinity of binding to 1–400, but an intact PTB domain was necessary for the binding. (D) Fine mapping the PTB binding site in DLC1. Myc-tagged tensin2 was co-transfected with a panel of FLAG-tagged DLC1 N-terminus fragments. FLAG-tagged fragments in the precipitates were detected by immunoblotting analysis with anti-FLAG antibody. Fragment 1–385 was the shortest fragment capable of being co-immunoprecipitated with tensin2. The band intensity in each lane was measured in the IP and Co-IP panels and the readings were normalized to lane 2. The relative Co-IP-to-IP ratios were also included.