Fig. 2.
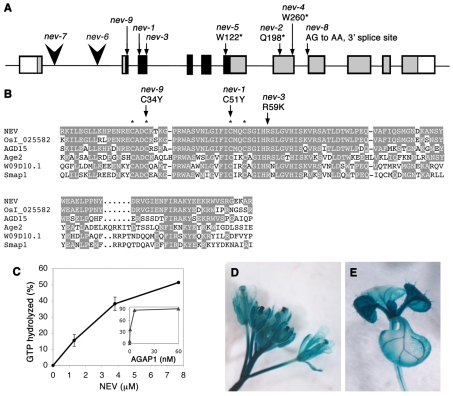
NEV encodes an ARF GAP. (A) The At5g54310 locus, showing the sites and sequence alterations of characterized nev mutations. Exons are shown as boxes, and the translated regions corresponding to the ARF-GAP domain and the rest of the corresponding protein are indicated in black and gray, respectively. Point mutations are marked by arrows, and T-DNA insertions by arrowheads. (B) Sequence alignment of the ARF-GAP domain from NEV and related proteins from plants (OsI_025582, AGD15), yeast (Age2), worm (W09D10.1) and mouse (Smap1). Amino acids conserved between NEV and other proteins are shaded, and the four cysteine residues that constitute the zinc finger are indicated with asterisks. The sites of nev missense mutations that affect two of these cysteines and a crucial arginine residue are marked by arrows above the alignment. Characterized ARF-GAP proteins in the alignment include yeast Age2 (Zhang et al., 1998) and mouse Smap1 (Sato et al., 1998). Uncharacterized proteins with closely related ARF-GAP regions include OsI_025582, AGD15 (Vernoud et al., 2003) and W09D10.1, predicted from Oryza sativa, Arabidopsis and Caenorhabditis elegans sequences, respectively. (C) NEV promotes GTP hydrolysis of mammalian ARF1. The ARF-GAP activity of recombinant full-length NEV protein was measured using AGAP1, a mammalian ARF-GAP, as a positive control (inset graph). The percentage of GTP bound to Arf1 that was converted to GDP is presented. The data are the summary of two experiments. (D,E) NEV regulatory regions direct broad expression of β-glucuronidase in flowers and shoot inflorescence stems (D), and in developing leaves and vascular strands (E).