Abstract
OBJECTIVES
In 2006, a new rotavirus vaccine was recommended for routine immunization of US infants. Because a previous rotavirus vaccine was withdrawn in 1999 after it was associated with intussusception, monitoring for this adverse event with the new vaccine is important. The objectives of this study were to assess intussusception hospitalizations trends among US infants for 1993 to 2004; provide estimates of hospitalization rates for intussusception for 2002–2004; and assess variations in background rates by age, race/ethnicity, and surgical management.
METHODS
By using the Healthcare Cost and Utilization Project’s State Inpatient Data-base that captures US hospital discharges from 16 states representing 49% of the birth cohort during 1993–2004 and from 35 states representing 85% of the birth cohort in 2002–2004, we examined hospitalizations among infants (<12 months of age) with an International Classification of Disease, Ninth Revision, Clinical Modification code for intussusception (560.0). Incidence rates were calculated by using census data, and rate ratios with 95% confidence intervals were calculated by using Poisson regression data.
RESULTS
Annual intussusception hospitalization rates declined 25% from 1993 to 2004 but have remained stable at ~35 cases per 100 000 infants since 2000. Rates were very low for infants younger than 9 weeks (<5 per 100 000) then increased rapidly, peaking at ~62 per 100 000 at 26 to 29 weeks, before declining gradually to 26 per 100 000 at 52 weeks. Compared with rates among non-Hispanic white infants (27 per 100 000), rates were greater among non-Hispanic black infants (37 per 100 000) and Hispanic infants (45 per 100 000); however, rates did not differ by race/ethnicity for infants who were younger than 16 weeks.
CONCLUSIONS
This assessment of US hospitalizations provides up-to-date and nationally representative prevaccine rates of intussusception. Because rates varied almost 12-fold by week of age and to a lesser extent by race/ethnicity during the age of vaccination, adjusting baseline rates to reflect the demographics of the vaccinated population will be crucial for assessing risk for intussusception after rotavirus vaccination.
Keywords: intussusception, rotavirus vaccine, vaccine safety monitoring
Rotavirus is the most common cause of severe gastroenteritis in US children who are younger than 5 years.1–3 In 1999, the first rotavirus vaccine (RotaShield; Wyeth Lederle Vaccines, Marietta, PA) licensed in the United States was withdrawn from the market <1 year after its introduction because of its association with intussusception. The risk for intussusception was elevated >20-fold in the 3- to 14-day period after the first dose of RotaShield, and a smaller increase in risk was also observed during the same period after vaccination with the second dose.4 In February 2006, a new rotavirus vaccine (RotaTeq; Merck, Whitehouse Station, NJ) was recommended for routine immunization of all US infants with 3 doses administered orally at 2, 4, and 6 months of age.5 In a prelicensure trial in which ~70 000 infants were immunized with either RotaTeq or placebo, no association was observed between the vaccine and intussusception during the 1- to 42-day period after vaccination6; however, postmarketing monitoring for intussusception after RotaTeq vaccination is necessary because of possible differences in the characteristics of infants who receive the vaccine in routine use compared with the clinical trials and the large numbers of infants being vaccinated.
The Vaccine Adverse Event Reporting System (VAERS) is an important tool for monitoring safety of vaccines used in the United States.7 Indeed, VAERS generated the first signal of the association between the previous rotavirus vaccine and intussusception.8 Because VAERS is a passive reporting system, the assessment of a potential relationship between reported adverse events and vaccination requires consideration of 3 important factors: completeness of reporting to VAERS, the number of vaccine doses administered, and the expected background incidence of the adverse event in the population that receives the vaccine. The last factor is particularly important for assessing data on intussusception reports to VAERS because the incidence of intussusception is known to vary substantially by age during the first few months of life9 and may vary by race/ethnicity and other demographic characteristics that might be related to access to vaccination. 10
Intussusception is a condition in which a segment of the bowel telescopes into a distal portion. It is the most common cause of intestinal obstruction among children, and most cases occur in infants who are <12 months of age.11–13 Approximately 1200 to 1400 cases of intussusception occur in US infants annually. The underlying cause of intussusception in most infants is unknown, but it has been associated with several pathogens, including adenoviruses.14–23 Previous assessments of intussusception rates among US infants have been based on National Hospital Discharge Survey data, which contain only a 0.5% to 1.0% sample of all US hospitalizations,10 hospital discharge data from a few selected states,10,24,25 or discharge data for groups that may not be representative of the general US population, such as Native Americans and children enrolled in managed care organizations. 10,26,27 Because intussusception is a relatively uncommon event and its epidemiology varies among different populations, a detailed analysis of a nationally representative sample is needed to assess disease trends and estimate incidence rates reliably. One previous study examined more representative data from 10 US states, but this study provided information only through 2001.9 Because studies from the United States,10,25 Australia,28 and Denmark29 noted declines in intussusception incidence during the 1990s, more up-to-date background information is needed to assess reliably any potential association between rotavirus vaccination and intussusception in the postlicensure era.
Our objective in this report was to examine hospital discharge data from US states between 1993 and 2004 to characterize in detail the epidemiology of intussusception hospitalizations among US infants and assess time trends. In particular, we provide up-to-date estimates of hospitalization rates for intussusception based on information from US community hospitals representing 85% of the US birth cohort for the most recent 3-year study period from 2002 to 2004.
METHODS
Data Sources
Hospitalization Data
We assessed the State Inpatient Database (SID) maintained by the Healthcare Cost and Utilization Project (HCUP) to perform a retrospective cross-sectional study of intussusception hospitalizations among infants who were <12 months of age from 1993 to 2004. HCUP is a partnership between state data organizations, hospital and other private data organizations, and the federal government to create a national information resource of patient-level health care data and is maintained by the Agency for Healthcare Research and Quality (AHRQ). SID contains nearly 100% of community hospital discharges, including discharges from pediatric hospitals for participating states. The 35 states participating from 2002–2004 represent 85% of the US birth cohort. This study was conducted in collaboration with HCUP staff at AHRQ.30,31
For this analysis, an intussusception diagnosis was defined as a hospitalization with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for intussusception (560.0) listed as a discharge diagnosis. The infant’s age in weeks, gender, race/ethnicity, state of hospital admission, length of stay, whether abdominal surgery was performed, and year of hospitalization were extracted from the discharge records. Age groups were defined on the basis of the recommended age windows during which the 3 vaccine doses are to be administered.
Population Data
Hospitalization rates by various demographic characteristics were generated on the basis of population data for participating SID states. For rate calculations, we used the National Center for Health Statistics’ bridged-race intercensal estimates of US children who were younger than 1 year for 1993 through 2004.32,33 When hospitalization rates by age were calculated, births were assumed to be evenly distributed throughout the year.
Analysis
Intussusception hospitalizations were examined by age in weeks, gender, race/ethnicity, and region. Rate ratios (RRs) with 95% confidence intervals (95% CIs) were calculated by using Poisson regression.
Trends in Incidence of Intussusception Hospitalizations From 1993 to 2004
To examine trends over time in intussusception hospitalizations during the entire study period, we restricted the analyses to 16 states (AZ, CA, CO, CT, FL, IA, IL, KS, MA, MD, NY, NJ, OR, SC, WA, and WI) that provided complete data for all years from 1993 to 2004. These 16 states represent ~49% of the US birth cohort. To examine trends by race and ethnicity, we further restricted the analysis to 9 states that coded race and ethnicity on ≥ 95% of discharge records for all years between 1993 and 2004 (CA, CT, FL, KS, MD, NJ, NY, SC, and WI). These 9 states represent ~35% of the US birth cohort.
Incidence and Epidemiology of Intussusception Hospitalizations in the Most Recent 3-Year Study Period, 2002–2004
To determine the rate of intussusception hospitalizations in the 3 years with most recent data preceding the implementation of the RotaTeq vaccine program, we studied data from 35 states (AZ, CA, CO, CT, FL, GA, HI, IA, IL, KS, KY, MA, MD, ME, MI, MN, MO, NC, NE, NJ, NV, NY, OH, OR, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, and WI) that provided complete data for all 3 years, 2002–2004. These 35 states represent ~85% of the US birth cohort. To examine the epidemiology of intussusception by race and ethnicity during this 3-year period, we restricted the analyses to the 16 states (CA, CT, FL, GA, KS, MA, MD, MO, NJ, NY, RI, SC, SD, TX, VT, and WI) that coded race and ethnicity on ≥ 95% of discharge records during the study period. These 16 states represent ~52% of the US birth cohort.
Age Analysis
To examine closely intussusception incidence by week of age, we included all intussusception hospitalization events recorded in any HCUP state for any year during 1993 to 2004. To avoid a possible age bias introduced by intussusception events associated with the previous RotaShield vaccine, we excluded data for the year 1999 and adjusted the denominator accordingly. The age-specific pattern in incidence was modeled using a spline curve fit to weekly estimates of intussusception incidence.
RESULTS
Trends in Incidence of Intussusception Hospitalizations During 1993 to 2004
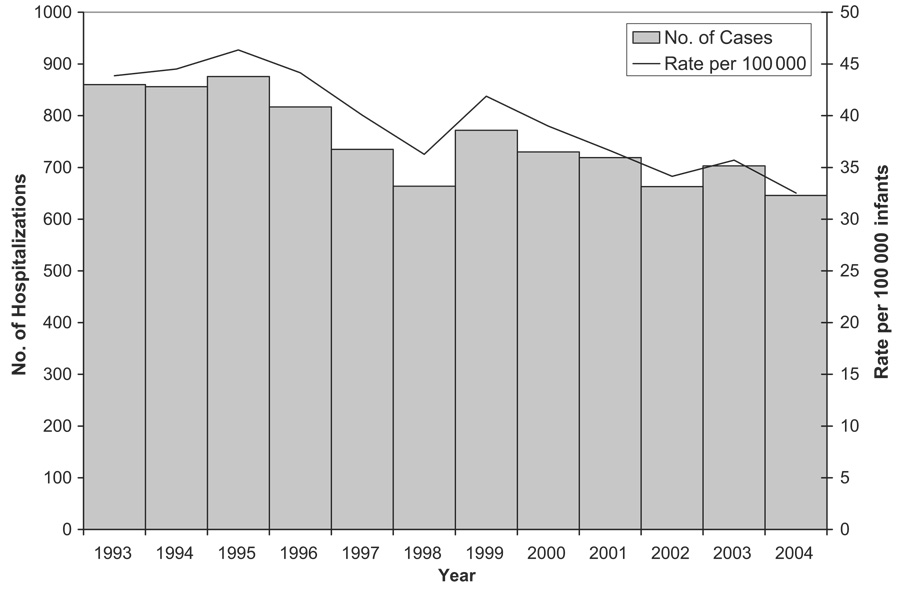
The overall rate of intussusception hospitalizations in infants who were <12 months of age declined by 25% during the 12-year study period, from 44 per 100 000 infants in 1993 to 33 per 100 000 infants in 2004 (P < .001; Fig 1). Most of this decline occurred during 1995 to 1998, and rates have remained relatively stable since 2000.
FIGURE 1.
Trends in intussusception hospitalization rates among infants <12 months of age based on Health Care Cost and Utilization Project data from 16 states, 1993–2004. Note that the 16 states include AZ, CA, CO, CT, FL, IL, IA, KS, MD, MA, NY, NJ, OR, SC, WA, and WI.
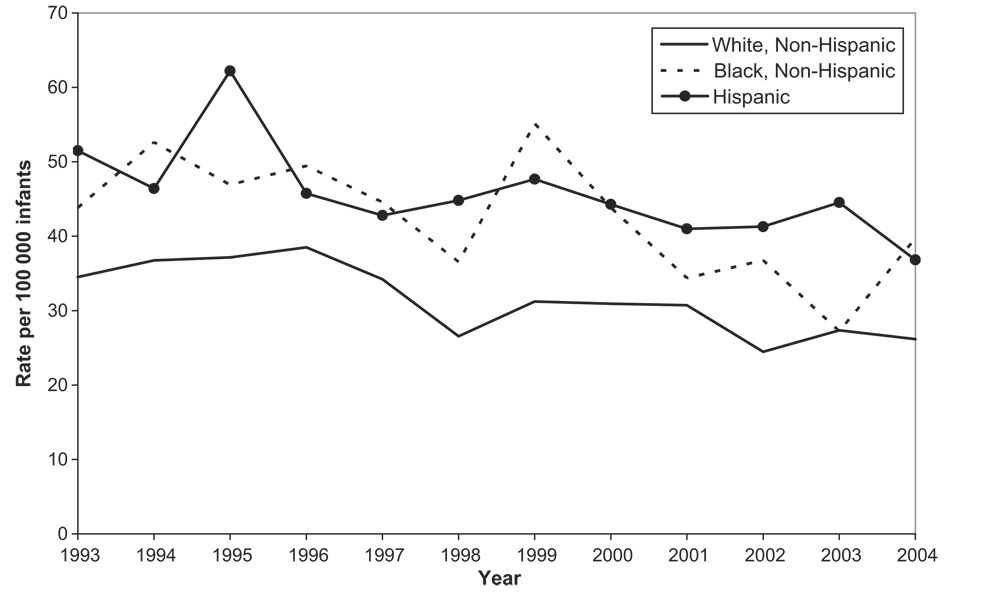
A similar decline in intussusception hospitalizations was observed among non-Hispanic white infants. In this group, rates declined by 26% from 35 per 100 000 infants in 1993 to 26 per 100 000 infants in 2004 (P < .004; Fig 2); however, rates among non-Hispanic black infants and Hispanic infants did not show any clear trend and fluctuated from year to year during the study period.
FIGURE 2.
Trends in intussusception hospitalization rates by race/ ethnicity among infants <12 months of age based on Health Care Cost and Utilization Project data from 9 states, 1993–2004. Note that the 9 states include CA, CT, FL, KS, MD, NJ, NY, SC, and WI.
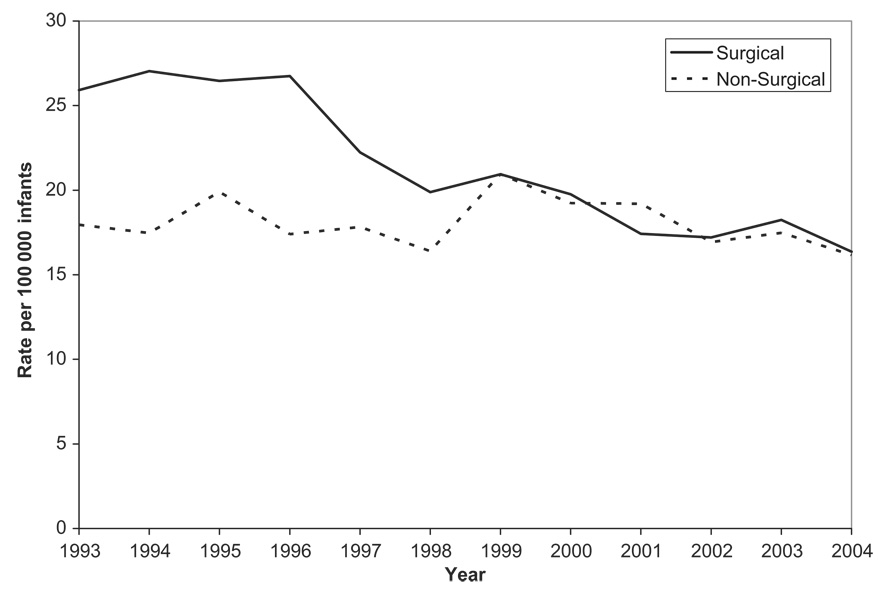
A gradual decrease in surgical intervention rates for intussusception from 59% of all intussusception cases during 1993–1995 to 51% during 2002–2004 (P < .0001) was noted. Overall, the rate of surgically treated intussusception declined by 38% during the study period, from 26 per 100 000 infants in 1993 to 16 per 100 000 infants in 2004 (P < .0001), whereas the incidence of nonsurgically managed intussusception remained steady at ~16 to 21 per 100 000 infants (Fig 3).
FIGURE 3.
Trends in rates of intervention for intussusception among infants <12 months of age based on Health Care Cost and Utilization Project data from 16 states, 1993–2004. Note that the 16 states include AZ, CA, CO, CT, FL, IL, IA, KS, MD, MA, NY, NJ, OR, SC, WA, and WI.
Incidence and Epidemiology of Intussusception Hospitalizations in the Most Recent 3-Year Study Period, 2002–2004
The average annual incidence of intussusception hospitalization for all infants who were younger than 1 year from 2002 to 2004 was 34 per 100 000 infants. Rates were ~50% higher for boys compared with girls (40 per 100 000 male infants vs 27 per 100 000 female infants; RR: 1.5; 95% CI: 1.4–1.6; Table 1). Compared with rates among non-Hispanic white infants (27 per 100 000), rates were greater among non-Hispanic black infants (37 per 100 000; RR: 1.4; 95% CI: 1.2–1.6) and Hispanic infants (45 per 100 000; RR: 1.7; 95% CI: 1.5–1.9). Rates also varied by region of the country, with the rates lowest in the Midwest (27 per 100 000 infants) compared with rates for the 3 other regions (36, 34, and 37 per 100 000 infants for the Northeast, South, and West, respectively).
TABLE 1.
Incidence of Intussusception Hospitalization Among Infants (<1 Year of Age) by Various Demographic Characteristics for 35 US States, 2002–2004
| Parameter | n | Rate per 100 000 Infants |
RR (95% CI) |
|---|---|---|---|
| Total | 3463 | 33.6 | |
| Gender | |||
| Male | 2097 | 39.8 | 1.5 (1.4–1.6) |
| Female | 1366 | 27.1 | Reference |
| Race/ethnicitya | |||
| White, non-Hispanic | 799 | 27.0 | Reference |
| Black, non-Hispanic | 356 | 37.0 | 1.4 (1.2–1.6) |
| Hispanic | 881 | 45.4 | 1.7 (1.5–1.9) |
| Asian or Pacific Islander | 73 | 20.1 | 0.7 (0.6–1.0) |
| Other | 202 | ||
| Age groups, wk | |||
| 1–5 | 43 | 4.3 | Reference |
| 6–14 | 207 | 11.6 | 2.7 (1.9–3.7) |
| 15–24 | 795 | 40.1 | 9.2 (6.8–12.6) |
| 25–32 | 860 | 54.3 | 12.5 (9.2–17.0) |
| 33–52 | 1557 | 39.3 | 9.1 (6.7–12.3) |
| Region of country | |||
| Northeast | 562 | 36.0 | 1.3 (1.2–1.5) |
| Midwest | 623 | 26.7 | Reference |
| South | 1264 | 34.4 | 1.3 (1.2–1.4) |
| West | 1014 | 37.2 | 1.4 (1.3–1.5) |
The median length of stay in hospital was 2 days (interquartile range: 1–4 days) and did not vary during the 3-year period. Of intussusception cases reported in 2002 to 2004, 51% required surgical intervention, and the proportion of cases that required surgery did not vary by age. The hospital fatality rate for intussusception hospitalizations was low, with only 0.4% of hospitalized cases resulting in death.
Age Analysis
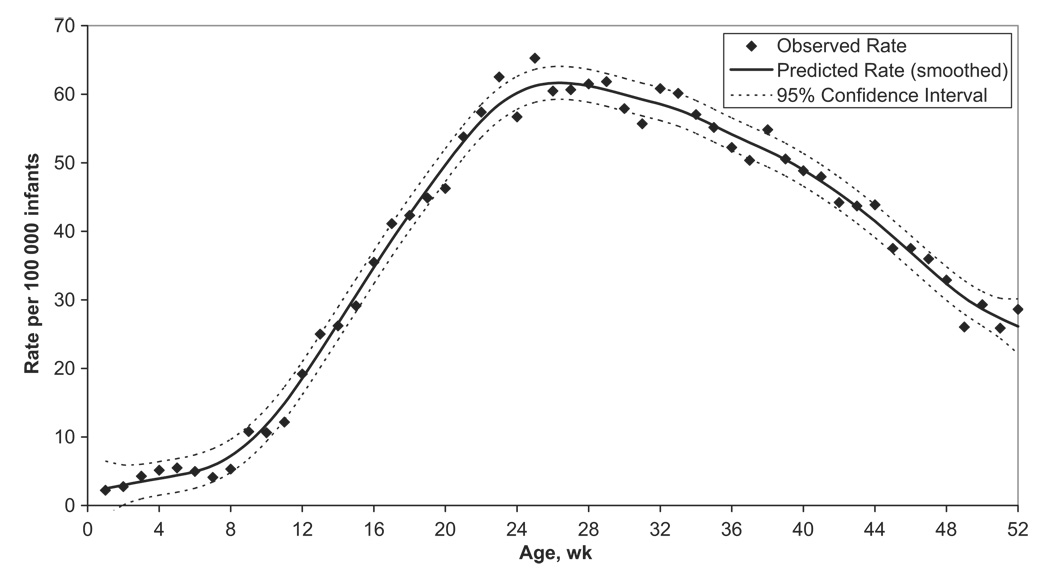
Rates of intussusception hospitalization varied substantially by age (Fig 4). Rates were low for infants who were younger than 9 weeks (rising from ~2 per 100 000 infants at birth to ~5 per 100 000 infants aged 8 weeks); then increased rapidly, peaking at ~62 per 100 000 infants for infants aged 26 to 29 weeks; and then declined later in infancy to 26 per 100 000 infants at 52 weeks of age.
FIGURE 4.
Intussusception hospitalization rates per 100 000 infants <12 months of age, by week of age. Analysis is based on all available records of intussusception hospitalizations from 39 Health Care Cost and Utilization Project states participating at least 1 year during 1993–2004. Note that the 39 states include AR, AZ, CA, CO, CT, FL, GA, HI, IL, IN, IA, KS, KY, ME, MD, MA, MI, MN, MO, NE, NV, NH, NJ, NY, NC, OH, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, and WI. Data from 1999 are excluded.
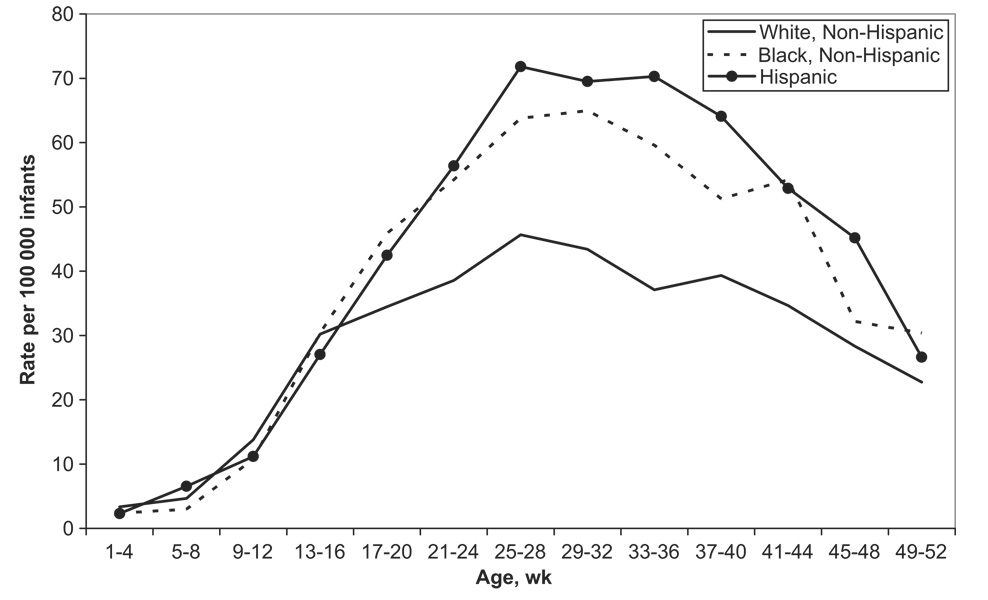
For infants who were younger than 16 weeks, intussusception rates did not vary meaningfully by race/ethnicity (Fig 5). This was in contrast to the 21- to 44-week age group, in which non-Hispanic white infants had substantially lower rates of intussusception compared with non-Hispanic black and Hispanic infants.
FIGURE 5.
Intussusception hospitalization rates by race/ethnicity and week of age among infants <12 months of age based on Health Care Cost and Utilization Project data from 12 states, 1997–2004. Note that the 12 states include CA, CT, FL, GA, KS, MD, MA, MO, NJ, NY, SC, and WI.
To illustrate the impact of variation in age-specific incidence of intussusception on estimates of background intussusception cases, we estimated the number of intussusception cases that would occur within 3 weeks of vaccination by chance alone if 1 million infants were vaccinated with 1 dose at specific ages during the recommended age at vaccination of 6 to 32 weeks (Table 2). The number of cases ranged from 3 if 1 million doses were given to infants 6 weeks of age to 36 if they were given to 1 million infants aged 27 weeks.
TABLE 2.
Number of Intussusception Cases Occurring by Chance During the 3 Weeks After Vaccination in a Hypothetical Scenario in Which 1 Million Infants Are Vaccinated by Varying Weeks of Age (Based on All 39 Participating HCUP States During 1993–2004, Excluding 1999 When RotaShield Was in Use)
| Age, wka | Predicted Rate (per 100 000 Infants) |
Expected No. of Cases During a 3-wk Period in 1 Million Vaccinated Infants |
|---|---|---|
| 6 | 4.9 | 3 |
| 9 | 9.2 | 5 |
| 12 | 18.6 | 11 |
| 15 | 30.6 | 18 |
| 18 | 42.5 | 25 |
| 21 | 53.1 | 31 |
| 24 | 60.2 | 35 |
| 27 | 61.6 | 36 |
| 30 | 59.9 | 35 |
| 33 | 57.7 | 33 |
Different scenarios under which all 1 million doses of vaccine are administered to children of a single age group.
DISCUSSION
This comprehensive assessment of intussusception hospitalizations among US infants provides several insights that are relevant for monitoring the safety of the new rotavirus vaccination program using VAERS data. First, we observed that incidence rates decreased during the beginning of the study period (1995–1998), but no discernible trends were observed in more recent years; therefore, our background rate estimates for 2002–2004 should be appropriate for comparison with rates observed after implementation of rotavirus vaccination. Second, we noted that intussusception rates varied considerably by week of age during the recommended 6- to 32-week age range for vaccination and that this factor had a major impact on estimates of the expected number of cases after vaccination. Our large sample size and representative data allowed us to estimate for the first time the incidence of intussusception by week of age during the first few months of life when incidence of intussusception increases rapidly. We can make precise estimates of the expected number of intussusception cases among vaccinated infants by chance alone by multiplying the weekly incidence of intussusception by the number of doses of vaccine administered to infants by week of age. Third, although race/ethnicity differences in intussusception rates among US infants have been previously reported,4,10 the HCUP data allowed us to explore this issue in more detail than previous reports. Similar to age, observed racial and ethnic differences in intussusception rates should be considered when estimating expected number of cases after vaccination, especially if the vaccine is distributed differentially among race/ethnic groups as suggested by different uptake rates in the public and private sector; however, because race/ ethnicity differences were less pronounced before 16 weeks of age, adjusting for these factors will have a lesser impact on estimation of expected number of cases after the first dose of vaccine than after the second and third doses.
We identified several other interesting epidemiologic features of intussusception hospitalizations among US infants that require additional study to explain them fully. First, the decline in overall intussusception rates observed during the beginning of the study period was largely attributable to a decline in cases that required surgical intervention, whereas the rate of nonsurgical treatment remained constant. Although this trend might reflect a true reduction in incidence of severe intussusception, it could also reflect changes in management practices, such as greater use of nonsurgical interventions that do not require hospitalization or increase in management through emergency departments or short-stay units to ease hospital crowding.34 Second, intussusception rates were very low and increased only slowly during the first 8 to 9 weeks of life but then increased very rapidly during the next few weeks. The reasons for this pattern are not clear but may be attributable to age-related milestones in the maturation of the intestinal lymphoid tissue or a decline in maternal antibodies that protect against infections that are associated with intussusception. Third, the racial and ethnic disparities in rates of intussusception hospitalizations were more pronounced after 4 months of age than in younger infants. Additional work is needed to understand the cause of this pattern, which may reflect differences in access to or timing of health care, particularly among older children, rates of breastfeeding, age of weaning or type of weaning foods, or other yet unrecognized factors.
Two important limitations of this study should be considered in interpreting the data. First, in this study of hospital discharge records, cases were identified using the intussusception-specific ICD-9-CM code, and no efforts were made to validate the diagnosis; therefore, it is possible that some true cases of intussusception might not be coded with the intussusception-specific ICD-9-CM code and that not all cases identified by this code are true cases of intussusception. This misclassification of cases might affect our rate estimates, and future studies to evaluate the diagnostic accuracy of the ICD-9-CM code for intussusception will be helpful. Second, these analyses capture only intussusception hospitalizations. Although almost all cases of intussusception likely come to medical attention, intussusception cases are increasingly being treated in noninpatient settings (eg, emergency department, short-stay or 23-hour observation admissions). One study of found that only 35% of patients who had intussusception and presented to a pediatric emergency department were hospitalized for treatment.34 Because these intussusception cases may not be captured by the HCUP inpatient database, we may have underestimated the true incidence of intussusception. The rate of surgical intervention observed for intussusception cases in this study (50%–60%) is greater than rates reported from studies (20%–25%) in Australia35 and Canada,36 which also suggested that inpatient discharge databases might capture only more severe cases of intussusception. Additional study of noninpatient intussusception cases such as those managed and discharged from emergency departments or short-stay units is needed to examine this issue fully and to adjust background rates as necessary.
CONCLUSIONS
This study provides information on recent trends in the incidence and epidemiology of intussusception hospitalizations in US infants that will be important for interpreting data on VAERS reports of intussusception after rotavirus vaccination.37,38 The observed age-related and race/ethnicity-related differences in background incidence, especially during the period when most infants receive the first dose of vaccine, highlight the need for close scrutiny of reports of intussusception to assess any causal association with vaccination. Other countries that have introduced rotavirus vaccines or are considering their use should consider similar assessments of the epidemiology of intussusception in their own setting to help with assessment of postmarketing safety monitoring data.
What’s Known on This Subject
Intussusception rates vary among different populations and may have declined in recent years. A previous rotavirus vaccine was withdrawn in 1999 due to its association with intussusception. With the introduction of a new rotavirus vaccine, monitoring intussusception rates is important.
What This Study Adds
This study provides up-to-date and nationally representative prevaccine baseline rates of intussusception hospitalization by age and race/ethnicity for infants <12 months of age that are important for monitoring the safety of the new rotavirus vaccine.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases.
This research was an intramural collaboration across 3 government agencies (AHRQ, Centers for Disease Control and Prevention, and National Institutes of Health) that involved analysis of complete intramural files; therefore, the study has higher data resolution (eg, more age groups) and data from more recent years than are currently available in the public domain. We acknowledge and thank the participating states, without whom the HCUP project would not be possible: Arkansas Department of Health and Human Services; Arizona Department of Health Services; California Office of State-wide Health Planning and Development; Colorado Health and Hospital Association; Connecticut—Chime, Inc; Florida Agency for Health Care Administration; Georgia—GHA: An Association of Hospitals and Health Systems; Hawaii Health Information Corporation; Illinois Department of Public Health; Indiana Hospital and Health Association; Iowa Hospital Association; Kansas Hospital Association; Kentucky Department for Public Health; Maine Health Data Organization; Maryland Health Services Cost Review Commission; Massachusetts Division of Health Care Finance and Policy; Michigan Health and Hospital Association; Minnesota Hospital Association; Missouri Hospital Industry Data Institute; Nebraska Hospital Association; Nevada Department of Human Resources; New Hampshire Department of Health and Human Services; New Jersey Department of Health and Senior Services; New York State Department of Health; North Carolina Department of Health and Human Services; Ohio Hospital Association; Oregon Association of Hospitals and Health Systems; Rhode Island Department of Health; South Carolina State Budget and Control Board; South Dakota Association of Healthcare Organizations; Tennessee Hospital Association; Texas Health Care Information Council; Utah Department of Health; Vermont Association of Hospitals and Health Systems; Virginia Health Information; Washington State Department of Health; West Virginia Health Care Authority; and Wisconsin Department of Health and Family Services.
Abbreviations
- VAERS
Vaccine Adverse Event Reporting System
- SID
State Inpatient Database
- HCUP
Healthcare Cost and Utilization Project
- AHRQ
Agency for Healthcare Research and Quality
- ICD-9-CM
International Classification of Disease, Ninth Revision, Clinical Modification
- RR
rate ratio
- CI
confidence interval
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
Reprints Information about ordering reprints can be found online: http://www.pediatrics.org/misc/reprints.shtml
REFERENCES
- 1.Charles MD, Holman RC, Curns AT, Parashar UD, Glass RI, Bresee JS. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993–2002. Pediatr Infect Dis J. 2006;25(6):489–493. doi: 10.1097/01.inf.0000215234.91997.21. [DOI] [PubMed] [Google Scholar]
- 2.Jin S, Kilgore PE, Holman RC, Clarke MJ, Gangarosa EJ, Glass RI. Trends in hospitalizations for diarrhea in United States children from 1979 through 1992: estimates of the morbidity associated with rotavirus. Pediatr Infect Dis J. 1996;15(5):397–404. doi: 10.1097/00006454-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rota-virus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117(6):1887–1892. doi: 10.1542/peds.2005-2351. [DOI] [PubMed] [Google Scholar]
- 4.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344(8):564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 5.Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999;48(RR2):1–23. [PubMed] [Google Scholar]
- 6.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Pool V, Iskander JK, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. MMWR Surveill Summ. 2003;52(1):1–24. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine—United States, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48(27):577–581. [PubMed] [Google Scholar]
- 9.Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. J Infect Dis. 2005;192 suppl 1:S36–S43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 10.Parashar UD, Holman RC, Cummings KC, et al. Trends in intussusception-associated hospitalizations and deaths among US infants. Pediatrics. 2000;106(6):1413–1421. doi: 10.1542/peds.106.6.1413. [DOI] [PubMed] [Google Scholar]
- 11.Stringer MD, Pablot SM, Brereton RJ. Paediatric intussusception. Br J Surg. 1992;79(9):867–876. doi: 10.1002/bjs.1800790906. [DOI] [PubMed] [Google Scholar]
- 12.DiFiore JW. Intussusception. Semin Pediatr Surg. 1999;8(4):214–220. doi: 10.1016/s1055-8586(99)70029-6. [DOI] [PubMed] [Google Scholar]
- 13.Roeyen G, Jansen M, Hubens G, Vaneerdeweg W, Eyskens E. Intussusception in infants: an emergency in diagnosis and treatment. Eur J Emerg Med. 1999;6(1):73–76. [PubMed] [Google Scholar]
- 14.Ross JG, Potter CW, Zachary RB. Adenovirus infection in association with intussusception in infancy. Lancet. 1962;2(7249):221–223. doi: 10.1016/s0140-6736(62)92315-2. [DOI] [PubMed] [Google Scholar]
- 15.Bell TM, Steyn JH. Viruses in lymph nodes of children with mesenteric adenitis and intussusception. Br Med J. 1962;2(5306):700–702. doi: 10.1136/bmj.2.5306.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner PS, Knox EG, Court SD, Green CA. Virus infection and intussusception in childhood. Br Med J. 1962;2(5306):697–700. doi: 10.1136/bmj.2.5306.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke EJ, Jr, Phillips IA, Alexander ER. Adenovirus infection in intussusception in children in Taiwan. JAMA. 1969;208(9):1671–1674. [PubMed] [Google Scholar]
- 18.Nicolas JC, Ingrand D, Fortier B, Bricout F. A one-year virological survey of acute intussusception in childhood. J Med Virol. 1982;9(4):267–271. doi: 10.1002/jmv.1890090404. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery EA, Popek EJ. Intussusception, adenovirus, and children: a brief reaffirmation. Hum Pathol. 1994;25(2):169–174. doi: 10.1016/0046-8177(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 20.Hsu HY, Kao CL, Huang LM, et al. Viral etiology of intussusception in Taiwanese childhood. Pediatr Infect Dis J. 1998;17(10):893–898. doi: 10.1097/00006454-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Bode CO, Omilabu SA. Viral isolates of intussusception in Nigerian infants. S Afr J Surg. 2002;40(2):57–58. [PubMed] [Google Scholar]
- 22.Guarner J, de Leon-Bojorge B, Lopez-Corella E, et al. Intestinal intussusception associated with adenovirus infection in Mexican children. Am J Clin Pathol. 2003;120(6):845–850. doi: 10.1309/LBRN-GF9M-JW2M-HT97. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj G, Kirkwood C, Bines J, Buttery J. Molecular epidemiology of adenovirus isolates from patients diagnosed with intussusception in Melbourne, Australia. J Clin Microbiol. 2006;44(9):3371–3373. doi: 10.1128/JCM.01289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang HG, Smith PF, Ackelsberg J, Morse DL, Glass RI. Intussusception, rotavirus diarrhea, and rotavirus vaccine use among children in New York state. Pediatrics. 2001;108(1):54–60. doi: 10.1542/peds.108.1.54. [DOI] [PubMed] [Google Scholar]
- 25.Simonsen L, Morens D, Elixhauser A, Gerber M, Van Raden M, Blackwelder W. Effect of rotavirus vaccination programme on trends in admission of infants to hospital for intussusception. Lancet. 2001;358(9289):1224–1229. doi: 10.1016/S0140-6736(01)06346-2. [DOI] [PubMed] [Google Scholar]
- 26.Chang EJ, Zangwill KM, Lee H, Ward JI. Lack of association between rotavirus infection and intussusception: implications for use of attenuated rotavirus vaccines. Pediatr Infect Dis J. 2002;21(2):97–102. doi: 10.1097/00006454-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Kramarz P, France EK, Destefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20(4):410–416. doi: 10.1097/00006454-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Justice F, Carlin J, Bines J. Changing epidemiology of intussusception in Australia. J Paediatr Child Health. 2005;41(9–10):475–478. doi: 10.1111/j.1440-1754.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 29.Fischer TK, Bihrmann K, Perch M, et al. Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics. 2004;114(3):782–785. doi: 10.1542/peds.2004-0390. [DOI] [PubMed] [Google Scholar]
- 30.HCUP Databases; Healthcare Cost and Utilization Project. [Accessed March 3, 2008];Rockville, MD: Agency for Healthcare Research and Quality; Overview of the State Inpatient Databases (SID) 2006 September; Available at www.hcup-us.ahrq.gov/sidoverview.jsp.
- 31.HCUP SID Database Documentation; Healthcare Cost and Utilization Project (HCUP) [Accessed March 3, 2008];Rockville, MD: Agency for Healthcare Research and Quality; SID Database Documentation. 2007 January; Available at www.hcup-us.ahrq.gov/db/state/siddbdocumentation.jsp.
- 32.National Center for Health Statistics. U.S. Census Population With Bridged Race Categories: Bridged-race intercensal estimates of the July 1, 1990-July 1, 1999, United States resident population by county, single-year of age, gender, race, and Hispanic origin, prepared by the U.S. Census Bureau with support from the National Cancer Institute. [Accessed March 3, 2008];2004 April 24; Available at www.cdc.gov/nchs/about/major/dvs/popbridge/popbridge.htm.
- 33.National Center for Health Statistics. U.S. Census Population With Bridged Race Categories: Estimates of the July 1, 2000-July 1, 2005, United States resident population from the Vintage 2005 postcensal series by year, county, age, sex, race, and Hispanic origin, prepared under a collaborative arrangement with the U.S. Census Bureau. [Accessed March 3, 2008];2006 August 16; Available at www.cdc.gov/nchs/about/major/dvs/popbridge/popbridge.htm.
- 34.Bajaj L, Roback MG. Postreduction management of intussusception in a children’s hospital emergency department. Pediatrics. 2003;112(6 pt 1):1302–1307. doi: 10.1542/peds.112.6.1302. [DOI] [PubMed] [Google Scholar]
- 35.Justice FA, Auldist AW, Bines JE. Intussusception: trends in clinical presentation and management. J Gastroenterol Hepatol. 2006;21(5):842–846. doi: 10.1111/j.1440-1746.2005.04031.x. [DOI] [PubMed] [Google Scholar]
- 36.Somme S, To T, Langer JC. Factors determining the need for operative reduction in children with intussusception: a population-based study. J Pediatr Surg. 2006;41(5):1014–1019. doi: 10.1016/j.jpedsurg.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration. FDA Public Health Notification: Information on RotaTeq and Intussusception. [Accessed March 3, 2008];2007 February; Available at www.fda.gov/cber/safety/phnrota021307.htm.
- 38.Centers for Disease Control and Prevention. Postmarketing monitoring of intussusception after RotaTeq vaccination—United States, February 1, 2006–February 15, 2007. MMWR Morb Mortal Wkly Rep. 2007;56(10):218–222. [PubMed] [Google Scholar]