Abstract
Naïve and memory CD4 and CD8 T cells represent a highly dynamic system with constant homeostatic and antigen-driven proliferation, influx, and loss of T cells. Thymic activity dwindles with age and essentially ceases in the later decades of life, severely constraining the generation of new T cells. Homeostatic control mechanisms are very effective to maintain a large and diverse subset of naïve CD4 T cells for many years up to the 8th decade of life, but eventually and abruptly fail at about the age of 75 years. In contrast, the CD8 T cell compartment is more unstable, with progressive diminution of naïve T cells and increasing loss of diversity already during mid adulthood. Vaccination strategies need to aim at developing a broad repertoire of memory T cells before the critical time period when the naïve CD4 T-cell repertoire collapses. Research efforts need to aim at understanding the homeostatic control mechanisms to ultimately expand the time period of repertoire stability.
Adaptive immune responses are initiated when T cells recognize endogenous antigen on activated and mature antigen-presenting cells. Successful responses depend on the repertoire of existing T cells (Nikolich-Zugich et al., 2004). The diversity of the T-cell compartment determines whether T cells are available that recognize antigen with high avidity. The frequency of each T-cell specificity in the existing repertoire influences how fast an initial immune recognition event can be amplified to generate a sufficient number of effector cells. Consequently, the naïve T-cell repertoire is highly diverse, providing the ability to recognize the universe of exogenous and potentially dangerous antigens. In contrast, the memory T-cell repertoire is selected by previous antigen exposure. Each memory T cell clone is present in larger frequency, guaranteeing a more rapid response upon re-exposure to the same antigen. Homeostatic regulation is important not only to control the size of the T-cell compartment, but also the composition of naïve and memory T cells, as well as the diversity of each T-cell compartment (Goldrath and Bevan, 1999a).
T cell homeostasis and age
The homeostatic control mechanisms have to deal with exogenous and endogenous challenges (Freitas and Rocha, 2000; Jameson, 2002). The T-cell compartment is a highly dynamic T-cell reservoir with sometimes conflicting kinetics of singular components that compete for space. The most evident example is the clonal expansion that occurs after naïve or memory T cells encounter antigen. This clonal expansion is dramatic and has been estimated to include 10 to 15 population doublings followed by equally rapid clonal contraction with a majority of expanded T cells dying again (Homann et al., 2001; Kaech et al., 2002; Murali-Krishna et al., 1998). It has been estimated that the surviving memory T-cell population has about a 1000-fold higher clonal size than naïve cells (Goronzy and Weyand, 2005). Antigen-specific stimulation, however, is not the only stimulus that drives T-cell kinetics. In the absence of exogenous antigen, memory and even naïve T cells exhibit a constant turnover. The daily replacement rate of T cells has been estimated to be on the order of 1%, which means that with about 3×1011 T cells in a human body, approximately 3×109 T cells have to be generated each day (Goronzy and Weyand, 2003; Hellerstein et al., 1999; Neese et al., 2002). Memory T cells have higher homeostatic proliferation than naïve T cells (Goronzy and Weyand, 2003), raising the question of whether they can out compete naïve T cells and, over time, significantly compromise the integrity of the naïve T-cell compartment.
Earlier data in mouse models have suggested that memory and naïve T cells do not compete for the same space (Tanchot and Rocha, 1995; Tanchot et al., 1997). However, the differences in regulatory control have remained poorly defined; regulatory control may slow down the demise of the naïve compartment, but certainly do not completely prevent it. It is, therefore, clear that age has a major impact on the composition of the T-cell compartment (Fagnoni et al., 2000; Naylor et al., 2005). It is important to emphasize that homeostatic mechanisms not only sustain the space of the T-cell compartment, but also the composition of functional subsets and ultimately the diversities and the distributions of different clonal sizes.
Thymic activity and age
If such a substantial number of new T cells are replaced every day, where do they come from? Obviously, new memory T cells are being shifted from the compartment of naïve T cells at the time when priming for a new exogenous antigen occurs. Also, homeostatic proliferation of naïve cells, if accelerated, can be associated with phenotypic transitions that are reminiscent of memory cell development. Naïve T cells can only be generated by two means, either by development from lymphocyte precursors and ultimately stem cells or by homeostatic proliferation where naïve T cells function as their own progenitor cells. T-cell development from a common lymphocyte precursor occurs, perhaps exclusively, in the thymus. At least since the 1960s it has been known that the amount of thymic tissue declines with age (Henry, 1967). Thymic epithelial space, which is the best biological correlate of functionally active tissues, declines from about 7 cm3 in the young adult to about 1 cm3 in the 55 to 65 year old. Recent studies have shown islands of functional tissue in the thymus of the elderly (Haynes et al., 2000; Steinmann et al., 1985; Steinmann, 1986); however, it is uncertain whether production of new thymocytes in these residual tissues is of quantitative importance.
In the absence of phenotypic markers specific for thymic emigrants, most studies of thymic output have relied on measuring T-cell receptor excision circles (TREC) (Douek et al., 1998; Kong et al., 1999). These signal-joint TRECs are formed during T-cell receptor (TCR)-α-chain rearrangement, when a substantial portion of the TCR δ locus is excised. Excised DNA is stored as episomes for an unknown amount of time in the newly generated T cell, but is not amplified during division. Frequencies of these episomes that can be quantified by RT-PCR provide an upper estimate of thymic output (Douek et al., 1998; Hazenberg et al., 2001; Kong et al., 1999). Studies by others and us have shown that the frequency of these TRECs declines exponentially with age (Douek et al., 1998; Koetz et al., 2000; Naylor et al., 2005). After the age of 50 to 60 years, TRECs can still be detected, but concentrations are minimal. From these data, it can be concluded that the generation of new T cells is minimal. Computer simulations have suggested that the decline in TRECs fits to a model with homeostatic proliferation being the only variable (Dutilh and de Boer, 2003). In this case, thymic activity would even cease at an earlier age and not significantly contribute to the regeneration of the naïve T-cell compartment starting from young adulthood. More direct data have come from studies of patients undergoing autologous bone marrow transplantation. In particular, Hakim et al have found that generation of naïve T cells is severely compromised after the age of 40 to 50 years (Hakim et al., 2005). These authors examined three different surrogate markers of thymic activity: increase in thymic size, repopulation with naïve T cells, and increases in frequency of TREC+ T cells. An excellent correlation was found between all three surrogate markers. However, only the exceptional patient older than 40 to 50 years of age showed an increase in any of these surrogate markers after bone marrow transplantation (Hakim et al., 2005). One could argue that these patients have undergone chemotherapy, which may have damaged the lymphocyte precursor cell pool or the thymic epithelial cells. However, these patients are clearly able to regenerate myeloid, NK, and B cells. Moreover, our studies in patients undergoing T-cell depletion with monoclonal antibodies that have no nonspecific effect on lymphoid cells have come to the similar conclusion that the ages between 40 and 50 years are watershed in terms of thymic function (Jendro et al., 1995).
Thus, naïve T cells face challenges on many different fronts. The naïve T-cell compartment progressively lacks the influx of newly developed T cells. Like any other cellular compartment, it is faced with the normal daily attrition. Moreover, antigen-specific recruitment and, possibly to some extent, homeostatic proliferation shift naïve T cells from the naïve compartment into the memory compartment. Finally, naïve T cells have to defend their space against invasion of memory T cells that have higher population kinetics due to chronic stimulation with persistent antigen and higher responsiveness to microenvironmental cues.
Differential homeostatic controls in CD4 and CD8 T cells
How is it that with these challenges the naïve T-cell compartment is maintained with age? Surprisingly, the answer is different for CD4 and CD8 T cells. Naïve T cells have been defined as CD45RA+ T cells that express the chemokine receptor CCR7 (Sallusto et al., 1999). As shown in Figure 1, the naive CD4 T-cell subset shows a modest decline with age (p=0.0002); however, in the majority of individuals the percentage of CD4 T cells expressing a naïve phenotype is still substantial at the age of 65 to 75 and only later on declines more rapidly. This data suggest that even in the absence of significant thymic influx, the size of the naïve CD4 T-cell compartment is sufficiently sustained for another two to three decades by homeostatic proliferation. The CD8 T-cell compartment shows a more rapid decline in the frequency of naïve T cells. By the age of 65 years, the naïve CD8 compartment is significantly shrunk (p=0.00000000002). The decreased percentage reflects a reduction in absolute numbers and not a relative reduction due to the expansion of the CD8 effector cell population. In fact, the CD4 to CD8 T-cell ratio is maintained up to the age of 75 years (data not shown). The mechanism of this more rapid decline in the CD8 compartment is not known. Both subsets should be equally affected by thymic inactivity. Beverley and colleagues have examined T-cell subset kinetics after labeling with deuterated glucose and have not found significant differences in the kinetics of CD4 and CD8 cells (Macallan et al., 2003; Wallace et al., 2004). Different growth factors may be involved in homeostatic proliferation of CD4 and CD8 T cells; however, if anything, these growth factors appear to be favoring CD8 cells (Jameson, 2002; Marrack et al., 2000; Sprent and Surh, 2003). Moreover, the survival of naïve CD8 T cells depends on the recognition of major histocompatibility complex MHC class I molecules, which are more abundantly expressed in the periphery of MHC class II molecules recognized by CD4 T cells (Ernst et al., 1999; Goldrath and Bevan, 1999b; Jameson, 2002). Interestingly, this difference between CD4 and CD8 T-cell homeostasis is not limited to the naïve T-cell compartment; memory T cells show similarly divergent behavior. During middle age, CD8 memory T cells are much more prone to develop an uneven repertoire with the expansion of clonal population. Moreover, phenotypic changes that have been attributed to replicative stress, such as the loss of expression of the CD28 molecule, are much more frequently found in the CD8 than in the CD4 compartment (Fagnoni et al., 1996; Vallejo et al., 1998).
Figure 1. CD8 T cells lose the naïve T-cell compartment more rapidly than CD4 T cells.
Peripheral blood mononuclear cells were examined by flow cytometry for the expression of CD4, CD8, CD45RA and CCR7, and the fractions of naïve CD4 and CD8 T cells were determined.
If CD4 T cells sustain a sufficient compartment size for an extended period of time, how long can they maintain their diversity and functional competence? Because of the high degree of TCR diversity, its assessment is very difficult. Theoretically, there should be more than 1015 different possible TCR rearrangements and α—β chain combinations; i.e., with a total size of the naïve T-cell compartment of about a hundred billion T cells, there is the theoretical possibility that each naïve T cell has its unique TCR (Davis and Bjorkman, 1988). This is highly unlikely because restrictions such as incompatible TCR α—β chain pairing or positive selection on MHC molecules limit the number of functional TCRs that are selected for populating the periphery. Moreover, such a diverse repertoire would be highly susceptible to instability over time. If each TCR would be initially present in only one copy, the loss of such a T cell would, with time, contract the TCR repertoire. Even naïve T cells are, therefore, present in identical replicates generated during and shortly after T-cell development in the thymus or in the periphery. Estimates of clonal sizes have come from the dilution of TRECs during thymic development (Douek et al., 1998; Okamoto et al., 2002). We have used TREC measurements in cord blood of babies born at different gestational ages (Schonland et al., 2003). These different studies have allowed estimating the clonal size of naïve T cells to involve about 100 cells.
T-cell receptor diversity and age
Arstila and colleagues have used TCR sequencing to estimate the clonal diversity of naïve CD4 T cells (Arstila et al., 1999). These investigators performed size separation of selected Vβ-Jβ TCR combinations, obtained all sequences of one particular size, and used this measurement to extrapolate on the total diversity of the T-cell compartment. They estimated that a young, healthy human individual has about 1-2×106 different TCR-β chains, each potentially paired with about 50 TCR-α chains. Naturally, this is a minimal estimate because the sample size of lymphocytes included in the analysis needs to substantially exceed the number of expected different TCRs in order not to miss any new sequences. We have used a different experimental approach to assess the diversity by randomly sequencing TCRs from naïve CD4 T cells and subsequently determining their frequency in a limiting dilution system of an independent blood sample of the same donor. TCR sequences in the limiting assay are detected by TCR V-Jβ-specific PCR followed by hybridization with TCR N-D-N-specific probes (Naylor et al., 2005; Wagner et al., 1998). These studies estimate that the naïve T-cell compartment includes about 20 million different TCR-β chains. Limiting dilution systems always provide an upper estimate because the assay system may not be sensitive enough to detect a single TCR-β chain. It is likely that the true diversity is in between those two results, in the range of several million TCR, with the average clonal size of a naïve T cell being about 500 cells. Both techniques came to the same conclusion that the TCR-β chain repertoire of memory cells is contracted approximately by a factor of 10. Each of these memory TCR-β chains is only paired with one to two α chains, suggesting an average clonal size of CD4 memory T cells of 100,000 cells.
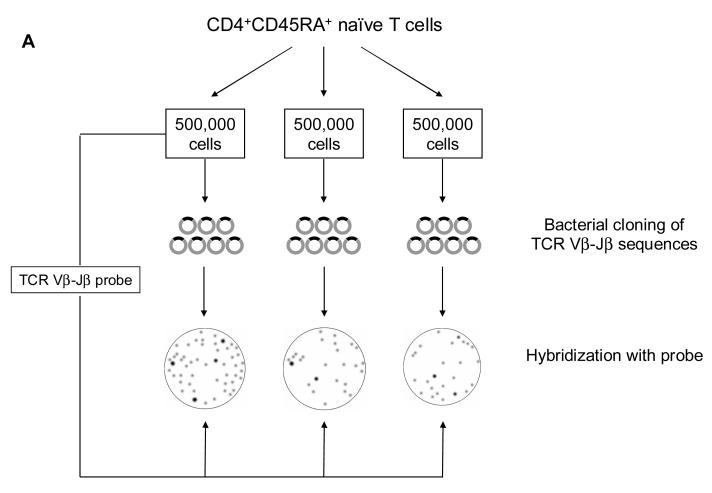
We have used these initial estimates of repertoire diversity to develop screening tools to assess repertoire diversity with age (Figure 2A). The likelihood that TCR sequences from one blood sample will be found in an independent blood sample from the very same patient should follow a Poisson distribution depending on the sample size of lymphocytes included in each. Hypothesizing that the true diversity is somewhere around 5 million different TCR-β chains, we elected sample sizes of 500,000 cells, collected three different samples of naïve cells from the blood of the same individual, and cloned all sequences from each sample after PCR with Vβ- and Jβ-specific primers. At the same time, a TCR Vβ-Jβ probe encompassing the N-D-N region from the first sample was generated. This probe was used to hybridize with all TCR colonies of the three different cloned samples. The basic assumption is that the TCR probe of sample 1 should hybridize with all of the bacterial clones from sample 1, but only with those bacterial clones in sample 2 and 3 that are also present in sample 1. If the cross-hybridization is limited, it can be expected that the diversity is significantly larger than the total number of lymphocytes included in the first sample. On the other hand, if cross-hybridization between unrelated samples is similar to the hybridization between probe and clones from the same sample, then the diversity is substantially lower than 500,000 different TCR-β chains. Figure 2B shows samples from individuals 20 to 30 years old compared to individuals older than 75. The young individuals clearly have a high degree of diversity; each new sample brings in additional TCR sequences that were not present in the initial sample. In contrast, the repertoire of all samples appears to be identical in the individuals older than 75, clearly indicating a contraction of the existing TCR-β chain repertoire (Naylor et al., 2005).
Figure 2. Contraction of naïve CD4 T-cell repertoire diversity with age.
A schematic overview of the experimental design is shown (A). CD4 naïve T cells were purified and divided into three samples of 500,000 cells each. cDNA of each sample was amplified with T-cell receptor BV8 and BJ2S5 primers and cloned using the TOPO TA Cloning Kit (Invitrogen). The amplified product of sample 1 was reamplified with internal primers to generate a short polyclonal probe representing the N-D-N sequences of sample 1. Bacterial colonies from all three samples were hybridized with the probe, and the fractions of hybridizing colonies were determined. Results from three individuals aged 20 to 30 years and older than 75 years are shown in Figure 2B. The high degree of cross-hybridization in the elderly population indicated a lack of T-cell receptor diversity. Reprinted with permission from (Naylor et al., 2005).
To more quantitatively examine changes in diversity with age, we used the limiting elution system in three individuals each from three different age strata, 25 to 30 years, 60 to 65 years, and 75 to 80 years (Figure 3). The results were surprising. As expected, the majority of naïve CD4 T cells in young adults were present in very low frequencies; i.e., T cells sharing one particular TCR-β chain were present only in small clonal sizes. A few naïve T cells were clonally expanded, either as a result of homeostatic proliferation or because they had been misclassified as naïve T cells. Surprisingly, this diversity picture did not change in the 60- to 65-year-old individuals, although these individuals must have had a long history of low to no thymic output and, therefore, hardly any influx of newly rearranged TCRs in the last two decades. If one assumes that naïve T cells turn over at least once per year and that most naïve cells have been seeded into the periphery by the age of 30 or maybe even earlier, then most naïve cells must have undergone more than 30 divisions by the age of 60. It is, therefore, striking how well the homeostatic mechanisms preserve TCR diversity. The picture dramatically changed by the age of 75 years. At that age, the population of infrequent T cells is essentially gone, and most T cells are present in clonal sizes of 1 million cells or larger. The number of different TCR-β chains in the repertoire is, therefore, severely restricted. Even if each of these TCR-β chains were paired with several different TCR-α chains, the naïve repertoire of a 75 year old would not be larger than the memory repertoire of a younger person. It is unclear what homeostatic mechanisms fail between the ages of 65 and 75, and what happens to the majority of naïve CD4 T cells. One possibility is that naïve T cells enter a state of senescence due to their extended replicative history. Indeed, Weng et al have shown telomeric erosion in naïve T cells with age (Weng et al., 1995). We have described that telomeres plateau at a relatively short length at about the age of 60 (Koetz et al., 2000). Telomeric erosion could therefore be responsible for a senescent phenotype of naïve T cells with reduced cell cycle progression or apoptosis. However, gene expression studies of naïve CD4 T cells in individuals around the age of 70 clearly showed that this is not the case (own unpublished observations). The gene expression profiles of such T cells are normal and not distinct compared to individuals in their third decade of life. In particular, naïve T cells do not show a gene expression fingerprint of senescence, but have normal expression of p53 and cell cycle inhibitors. Moreover, such T cells also do not have a functional defect and are fully competent to proliferate in response to antigen presented by dendritic cells.
Figure 3. The age period between 65 and 75 years is critical for CD4 T-cell repertoire contraction.
T-cell receptor sequences were identified in a sample of naïve CD4 T cells, and their frequencies were determined in an independent sample from the same individual by limiting dilution followed by BV-BJ amplification and hybridization for N-D-N sequences as described (Naylor et al., 2005; Wagner et al., 1998). Low frequencies of the given T-cell receptor sequences indicate a highly diverse repertoire. The results show that contraction of naïve CD4 T-cell diversity with age is a non-linear process abruptly occurring between the ages of 65 and 75. Modified from (Naylor et al., 2005) with permission.
Although these experiments did not provide evidence for cellular senescence in 70- to 75-year-old individuals, naïve CD4 T cells may have reached their upper lifespan at this age. In support of this hypothesis, most CD4 T cells that express the phenotype of naïve T cells appear to be masqueraded memory T cells. More than one-third of the TCR sequences derived from phenotypically defined naïve CD4 T cells were found to be shared with memory T cells in individuals older than 75 years. Specifically, out of about 100 TCR sequences for a particular Vβ-Jβ combination, one quarter were identified both in the naïve compartment and the memory compartment of CD4 T cells. Such a sharing of TCR sequences was the rare exception in individuals younger than 65, documenting that only up to this age population is the phenotypic distinction of naïve and memory CD4 T cells accurate (Naylor et al., 2005).
The likely interpretation of this finding is that between the ages of 65 to 75 years, homeostatic mechanisms fail in the naïve compartment, resulting in a rapid loss of T cells. This failure appears to be intrinsic—and not secondary—to competitive exclusion by CD8 or CD4 memory cells. It is likely that the masquerading of CD4 memory cells as naïve cells and the invasion of these cells into the naïve CD4 compartment is a secondary phenomenon to fill the space left by the naïve CD4 T cells.
Synopsis and outlook
These observations on the influence of age on T-cell homeostasis have implications for understanding immune defects in the elderly and planning vaccine strategies. The immune repertoire of 75-year-old individuals appears to be severely compromised (Naylor et al., 2005). Such individuals have to rely on memory T-cell responses and are unlikely to be able to yield primary T-cell responses. Vaccination strategies, therefore, have to aim at establishing a broad pool of memory T cells before individuals enter this age. Before this age, CD4 cells and CD8 cells appear to behave very differently, although they should be equally affected by the complete demise of thymic function in mid-adulthood. Although TRECs can be found at low frequency even during old age and histological evidence for isolated functional thymic epithelial clusters exists, cumulative evidence suggests that thymic activity after the ages of 40 to 50 years cannot be sufficiently restored to rebuild a repertoire and that even before that time influx of new thymic immigrants is minimal. The slow but certain decline of the CD8 naïve T cell compartment is, therefore, not unexpected. However, it is most surprising how well the CD4 T cell compartment is maintained both in numbers and in diversity. Functional data on naïve CD4 T cells from individuals between the ages of 60 to 70 also indicated that these T cells are fully competent and do not have signaling or proliferative defects. Understanding these successful homeostatic mechanisms and their eventual failure after the ages of 70 to 75 years of age is, therefore, of utmost importance. Interventions aimed at extending this stable equilibrium for several more years may be the most promising and effective way to improve immune competence in the elderly.
Acknowledgment
The authors thank Tamela Yeargin for manuscript editing.
Sources of Support: This work was funded in part by grants from the National Institutes of Health (RO1 AI 57266 and R01 AG 15043).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Dutilh BE, de Boer RJ. Decline in excision circles requires homeostatic renewal or homeostatic death of naive T cells. J Theor Biol. 2003;224:351–358. doi: 10.1016/s0022-5193(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999a;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999b;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, Gress RE. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med. 2001;79:631–640. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune JM. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- Henry L. Involution of the human thymus. J Pathol Bacteriol. 1967;93:661–671. doi: 10.1002/path.1700930227. [DOI] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- Jendro MC, Ganten T, Matteson EL, Weyand CM, Goronzy JJ. Emergence of oligoclonal T cell populations following therapeutic T cell depletion in rheumatoid arthritis. Arthritis Rheum. 1995;38:1242–1251. doi: 10.1002/art.1780380912. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong FK, Chen CL, Six A, Hockett RD, Cooper MD. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci U S A. 1999;96:1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macallan DC, Asquith B, Irvine AJ, Wallace DL, Worth A, Ghattas H, Zhang Y, Griffin GE, Tough DF, Beverley PC. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–2326. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]
- Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer BC, Swanson B, Kappler J. Homeostasis of alpha beta TCR+ T cells. Nat Immunol. 2000;1:107–111. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Neese RA, Misell LM, Turner S, Chu A, Kim J, Cesar D, Hoh R, Antelo F, Strawford A, McCune JM, Christiansen M, Hellerstein MK. Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A. 2002;99:15345–15350. doi: 10.1073/pnas.232551499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Douek DC, McFarland RD, Koup RA. Effects of exogenous interleukin-7 on human thymus function. Blood. 2002;99:2851–2858. doi: 10.1182/blood.v99.8.2851. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schonland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, Weyand CM. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–1434. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- Sprent J, Surh CD. Cytokines and T cell homeostasis. Immunol Lett. 2003;85:145–149. doi: 10.1016/s0165-2478(02)00221-3. [DOI] [PubMed] [Google Scholar]
- Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Rocha B. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+ T cell pools. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Rosado MM, Agenes F, Freitas AA, Rocha B. Lymphocyte homeostasis. Semin Immunol. 1997;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J Biol Chem. 1998;273:8119–8129. doi: 10.1074/jbc.273.14.8119. [DOI] [PubMed] [Google Scholar]
- Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Zhang Y, Ghattas H, Worth A, Irvine A, Bennett AR, Griffin GE, Beverley PC, Tough DF, Macallan DC. Direct measurement of T cell subset kinetics in vivo in elderly men and women. J Immunol. 2004;173:1787–1794. doi: 10.4049/jimmunol.173.3.1787. [DOI] [PubMed] [Google Scholar]
- Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]