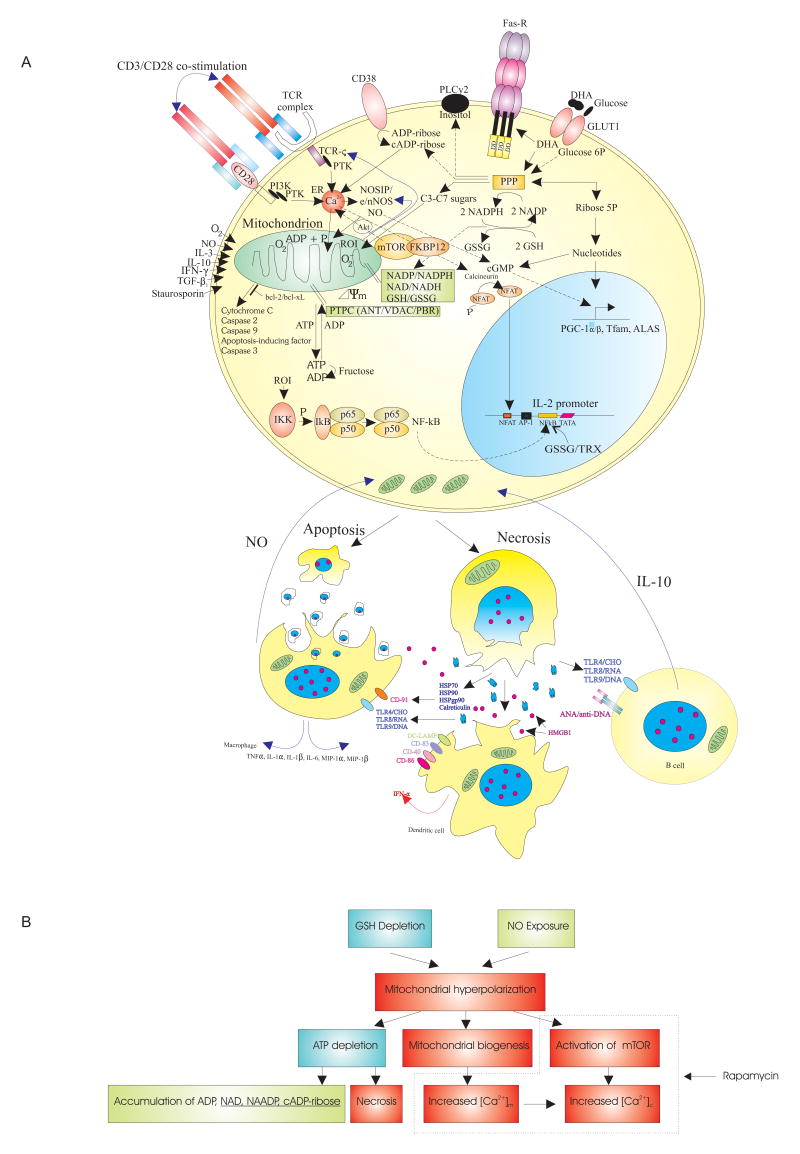
Fig 1.
A) Schematic outline of the metabolic pathways controlling (PPP,GSH, NO) and sensing (mTOR) mitochondrial hyperpolarization (MHP) of lupus T cells. In normal T cells, MHP and mitochondrial biogenesis is mediated via production of NO by eNOS or nNOS (28)and up-regulation of transcription factors PGC-1α, Tfam, and ALAS (39). NO production by eNOS may be compartmentalized to the T cell synapse (29). NO causes transient MHP via reversible inhibition of complex IV/cytochrome c oxidase (7) and persistent MHP via S-nitrosylation of complex I of the ETC in a state of GSH depletion (24). The PPP regulates the Δψm by producing 1) NADPH that serves as a reducing equivalent for GSH regeneration from its oxidized form GSSG and for production of NO by NOS and 2) ribose 5-phosphate for biosynthesis of nucleotides, ADP, ATP, NAD, NAADP, (c)ADP-ribose, and cGMP, the latter is a second messenger of NO. NAADP and (c) ADP-ribose induce Ca2+ release from the endoplasmic reticulum (ER) via ryanodine receptors (RyR). mTOR senses Δψm and regulates IP3R-mediated Ca2+ release (40). The intracellular rapamycin receptor FKBP12 directly binds the RyR. The Bcl-2 family proteins control permeability of the outer mitochondrial membrane and release of apoptosis-inducing factors. Necrosis-prone T cells release oxidized DNA and HMGB1 which stimulate B cells, macrophages, and dendritic cells (DC). In turn, B cells produce IL-10 and macrophages and DC produce NO which stimulate MHP of T cells. B) Proposed hierarchy of metabolic pathways upstream and downstream of MHP in lupus T cells. Broken line demarcates checkpoints affected by rapamycin.