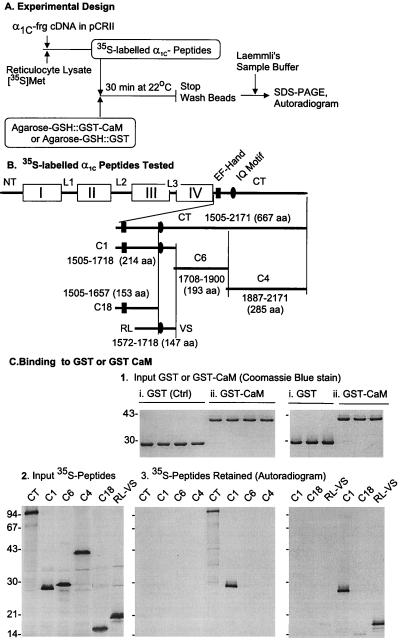
Figure 1.
Identification of the RL–VS segment of the α1C C terminus as an attachment site for CaM. (A) The strategy of the experiments. frg, fragment; [35S]Met, [35S]methionine. (B) Diagram of main structural motifs of α1C and placement of C-terminal fragments in relation to each other. I–IV, homologous repeat sequences forming transmembrane domains of the Ca2+ channel; NT, N terminus; L1–L3, connecting loops; CT, C terminus. (C) Only RL–VS containing fragments of the C terminus bind CaM. Molecular mass markers are expressed in kDa. (C, 1) Input GST or GST-CaM visualized by Coomassie blue staining of polyacrylamide gels resulting from the electrophoresis of proteins eluted from Agarose-GSH∷GST or GST-CaM that had been incubated with [35S]α1C fragments (partial view). (C, 2) Autoradiogram of SDS/PAGE analysis of in vitro translated α1C fragments tested for CaM-binding activity. (C, 3) Autoradiogram of the gel shown in C, 1.